Abstract
Endometrial carcinomas arising in a bicornuate uterus are rare, only five case of which have been previously reported. We present a case of endometrial cancer arising in a bicornuate uterus, occurring in a 65-year-old woman. Unlike previously reported cases, our case showed mixed endometrial adenocarcinoma and undifferentiated carcinoma in one horn and focal adenocarcinoma in the other. Adequate tissue sampling of both horns is necessary for accurate diagnosis of malignancy in patients with a bicornuate uterus. Physicians should be aware of the possibility of this abnormality in cases when endometrial cancer is suspected but histology fails to confirm.
The uterine endometrium and myometrium are formed secondary to fusion of the paired mullerian (paramesonephric) ducts during embryogenesis. Fusion defects of the mullerian ducts cause duplication of uterus and/or the vagina. If the inferior portions of mullerian ducts fail to fuse, a double uterus (didelphys) develops. The lack of fusion in superior portions of mullerian ducts result in a bicornuate uterus usually with a septate vagina [1]. If the ducts fuse but the wall between the two lumens do not degenerate, it results to abnormal septate uterus. When the fusion defect is confined to the fundus, the anomaly is called arcuate uterus [2]. Although the prevalence of mullerian duct anomalies is 1% and 3% in women with normal fertility and women with repeated pregnancy loss, respectively [3], the incidence is underestimated due to their asymptomatic nature.
Endometrial carcinoma associated with bicornuate uterus, especially the occurrence of cancer in both horns is rarely reported [4,5,6,7,8], and correlation between uterine anomaly and risk of endometrial cancer has never been shown. In this report, we describe a case of mixed endometrial adenocarcinoma and undifferentiated carcinoma arising in one horn with focal adenocarcinoma in the other horn of a bircornuate uterus.
A 65-year-old obese woman (gravida 3 and para 2) presented to a private clinic with a complaint of vaginal bleeding for 3 months, having been menopausal since age 55. The result of Pap smear was atypical glandular cells, favor neoplastic with negative HPV (human papillomavirus) DNA test. The patient had history of hypertension and hyperlipidemia. Her medications included estrogen replacement therapy and aspirin for 4 years.
She was referred to the Yonsei University Wonju Severance Christian Hospital for definitive diagnosis and therapy. Abdominal computed tomography revealed uterus didelphys with complete duplication of uterine horn and cervices and suspicious 2.0×1.7-cm-sized, oval-shaped relatively soft tissue mass in right cervix. Abdominal ultrasonography result showed mild fatty liver change with two hepatic cysts in S6 (0.7 cm) and S7 (2.2 cm). PET-CT (positron emission tomography-computed tomography) revealed focal FDG (fludeoxyglucose) uptakes in the left uterine cavity.
Uterine endometrial and cervical biopsies were performed. The endometrial biopsy revealed malignant tumor, suggestive of undifferentiated carcinoma while cervical and endocervical biopsies showed inflammatory change.
Total abdominal hysterectomy, bilateral salpingo-oophorectomy, and staging workup (bilateral pelvic lymph node dissection and pelvic washings) were performed. At the time of surgery, it was determined that the patient had a bicornuate uterus. Gross examination revealed a bicornuate uterus (Fig. 1A) (left horn 6.5×4×3.5 cm, right horn 7×4×3 cm) with a single cervix (4×3.5×3 cm). On opening, the entire endometrium of left horn showed an ill-defined white-gray granular mass, measuring about 6×4×0.5 cm (Fig. 1B). The endometrium of right horn was grossly unremarkable with focal granular irregularity in the lower uterine segment. The myometrium revealed two small leiomyomas in the right horn, measuring 2 cm in diameter in the larger one. The attached bilateral ovaries and tubes showed no gross abnormality.
Microscopic examination revealed a mixed endometrioid adenocarcinoma (80% component) (Fig. 1C) and undifferentiated carcinoma (20% component) (Fig. 1D) in the left endometrium. The endometrioid adenocarcinoma had less than 50% solid areas, hence designated as International Federation of Gynecology and Obstetrics (FIGO) grade II. Immunohistochemical stains showed positivity for EMA, CAM 5.2, estrogen and progesterone receptors in endometrioid adenocarcinoma component while undifferentiated carcinoma component showed no positivity (Fig. 2). The Immunohistochemical stain for cytokeratin showed diffuse positivity in endometrioid adenocarcinoma component and focal positivity in undifferentiated carcinoma component (Fig. 2). The tumor showed superficial invasion (0.4 cm in maximum depth) into the myometrium (2.5 cm in thickness). The endometrium of right horn showed a tiny focus of endometrioid adenocarcinoma, measuring less than 1 mm in diameter. The bilateral adnexae, parametria and pelvic lymph nodes were all free from tumor. The disease was consistent with FIGO stage IA (T1aN0Mx). Other histological findings were adenomyosis and leiomyomas in myometrium. The patient recovered from surgery and is receiving chemoand radiotherapy.
To the best of our knowledge, this is the first report of a mixed endometrioid adenocarcinoma and undifferentiated carcinoma in a bicornuate uterus with a focal endometrioid adenocarcinoma in other horn. Although Mullerian duct anomalies have not shown increased risk of cervical, endometrial and ovarian cancers, a few reports of endometrial cancers arising in bicornuate uterus have been reported [4,5]. Most reports showed endometrial adenocarcinoma in one horn [5,6] and only one case reported the endometrial adenocarcinoma involving both horns [4]. While all reports showed endometrioid adenocarcinoma, one report showed mixed endometrioid adenocarcinoma and serous papillary carcinoma [6].
The immunohistochemical stain profile of endometrial undifferentiated carcinoma includes focal or weak cytokeratin and EMA positivity and estrogen, progesterone receptor negativity [2]. Since our case was negative for estrogen and progesterone receptors, EMA and CAM 5.2 and focal positive for cytokeratin in undifferentiated component, while the endometrioid component was positive for all markers, the undifferentiated component was counted as the secondary component of the tumor.
Most of the endometrial cancers are estrogen-dependent endometrioid types and small percentage shows other aggressive, poorly differentiated non-estrogen dependent types. Estrogens are known as regulators of homeobox (HOX) gene expression, which is necessary for the development of normal reproductive tract. Abnormalities in HOX gene expression through abnormal molecular signaling of estrogen receptor result in mullerian duct abnormalities in mice [4,9]. Mullerian duct anomalies could be protective against endometrial carcinoma through abnormal estrogen receptor signaling. However, in the present case both estrogen-dependent and non-estrogen dependent types of endometrial cancers were found.
The possibility of uterine abnormalities should be included in the differential diagnosis of postmenopausal bleeding. Because of its asymptomatic nature, the prevalence of these anomalies can be higher than reported especially in women with normal fertility. In our case, the patient had three pregnancies and uterine anomaly was diagnosed secondary to the cancer screening. Furthermore, although patient had cancer in both horns, cancer foci in one horn was significantly small, raising the question that if the biopsy was taken from that horn, the diagnosis and treatment could be delayed. In the presence of persistent postmenopausal bleeding and a negative biopsy, a pelvic ultrasound should be considered.
In summary, we present an interesting case of a mixed endometrioid adenocarcinoma and undifferentiated carcinoma arising in one horn of a bicornuate uterus with a focal endometrioid adenocarcinoma in the other horn.
Figures and Tables
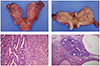 | Fig. 1The gross appearance of a bicornuate uterus. (A) External surface of the bicornuate uterus. (B) On opening, the endometrium of left horn shows diffuse granular polypoid mass. Microscopic findings of the case. (C) Microscopic findings of mixed endometrioid adenocarcinoma (left) and undifferentiated carcinoma (right) of left horn. (D) A tiny focus of endometrioid adenocarcinoma in right horn (H&E, ×100). |
References
1. Gilbert-Barness E. Potter's pathology of the fetus, infant, and child. 2nd ed. Philadelphia: Mosby Elsevier;2007.
2. Blaustein A, Kurman RJ, Ellenson LH, Ronnett BM. Blaustein's pathology of the female genital tract. 6th ed. New York: Springer;2011.
3. Troiano RN, McCarthy SM. Mullerian duct anomalies: imaging and clinical issues. Radiology. 2004; 233:19–34.
4. Shirley S, Devi VS, Krishnamurthy R, Nabhi MV, Majhi U, Selvaluxmy G. Endometrial adenocarcinoma involving both horns of a bicornuate uterus. J Cancer Res Ther. 2010; 6:304–306.
5. Dane C, Tatar Z, Dane B, Erqinbas M, Cetin A. A single horn endometrial carcinoma of a uterus bicornis unicollis. J Gynecol Oncol. 2009; 20:195–197.
6. Moore JR, Davidson SA, Singh M. Endometrial carcinoma in one horn of a bicornuate uterus. Gynecol Oncol. 2004; 95:729–732.
7. Herzog TJ, Fry R, Husseinzadeh N. Adenocarcinoma in a single horn of a bicornuate uterus. A case report. J Reprod Med. 1991; 36:619–621.
8. Kondi-Pafiti A, Spanidou-Carvouni H, Dimopoulou C, Kontogianni CI. Endometrioid adenocarcinoma arising in uteri with incomplete fusion of Mullerian ducts: report of three cases. Eur J Gynaecol Oncol. 2003; 24:83–84.
9. Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing müllerian system. FASEB J. 2000; 14:1101–1108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download