Abstract
Factor VII (FVII) deficiency is an infrequent hereditary bleeding disorder that can make excessive bleeding in surgical interventions, such as a postpartum hemorrhage in a cesarean section. Although a recombinant form of activated FVII has been applied for bleeding control in FVII-deficient patients, its applications in the field of obstetrics are still limited, especially in Korea. Replacement of blood products is still preferred as first-line therapy, with antifibrinolytic agents used as adjunctive therapy. We report herein the case of a successful cesarean section in an 18-year-old woman with FVII deficiency.
The substantial risk of excessive bleeding in patients during cesarean section is well known. Meticulous attention is required in the preoperative and postoperative treatment of patients with coagulation disorders. Factor VII (FVII) deficiency is an infrequent autosomal recessive bleeding disorder that has a prevalence of 1 in 500,000 individuals without racial or gender preference [1]. The molecular defect consists of mutations in the gene encoding for FVII, the protein that initiates the extrinsic coagulation pathway [2]. There are 26 recognized patients (14 women) with congenital FVII deficiency in Korea [3]. FVII deficiency is the only genetic coagulation disorder that has an isolated prolongation of the prothrombin time (PT) with a normal activated partial thromboplastin time (aPTT). However, FVII deficiency is seen more often in patients with chronic liver disease and in those who use warfarin or Coumadin. It is also common in patients with vitamin K deficiency due to long-term use of antibiotics and in those with bile duct obstruction or poor intestinal absorption. Careful medical history taking, a factor assay, and genotyping for FVII are required to confirm the diagnosis. The prevalence rate of surgical bleeding in these patients is approximately one-third [4]. Replacement therapy should be applied in patients undergoing surgery who have a history of recurrent bleeding and an FVII activity level of less than 10% to 15% [5]. There are many reports on the risk of bleeding in surgical procedures and the appropriate perioperative management in patients with FVII deficiency [6]. However, to the best of our knowledge, this is first case report on the postpartum management of maternal FVII deficiency in Korea. There has been only one case report about the management of delivery in a patient with a bleeding disorder in Korea, but it was a vaginal delivery and the patient had congenital factor V deficiency [7]. We review a case of cesarean section in a patient with FVII deficiency at our hospital and delineate the efficacy of management by fresh frozen plasma (FFP) and antifibrinolytic agents.
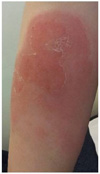
The patient was 18 years old, gravida 1, para 0. Menstruation was irregular, and its duration was 5 to 7 days with a moderate amount of bleeding. She had no past surgical history but a number of dental extractions. She was referred to our hospital at 31+1 weeks of gestation due to known FVII deficiency. At 4 years old, she underwent a dental extraction and had prolonged, but not acute, unusual bleeding. She was referred to a pediatrician, and FVII deficiency was suspected based on hemostatic screening, which revealed an isolated prolongation of PT with a normal aPTT. A Factor assay to evaluate FVII activity (4%) confirmed the diagnosis. Factor II, V, and X were normal. Her family had no history of bleeding disorders. Genotyping for FVII was recommended, but her family declined due to financial concerns. After FVII deficiency was diagnosed, the patient had two times more dental extractions. She received FFP before each procedure to prevent excessive bleeding and she did not have any unusual bleeding. During the pregnancy, there was no vaginal spotting in the first trimester. Sonographic findings were normal and the fetal growth rate was appropriate for the gestational age. Breech presentation was noted for all prepartum evaluations. She was admitted to the hospital at 39+0 weeks of gestation for cesarean delivery due to the persistent breech presentation. FVII activity and PT had been measured several times during her lifetime and always ranged between 1.56% and 11% and 2.21 and 8.09, respectively (Fig. 1). The result of the FVII assay in a prenatal test at 34+1 weeks of gestation was 7.00%. Upon discussion with a hematologist, we decided to infuse three units of FFP just before and continuously during the surgery. A cesarean section was performed on the second day of hospitalization under general anesthesia with infusion of FFP and successfully executed without unusual bleeding. The fetus was male, weighed 3,130 g, had no congenital anomalies, and the 1- and 5-minute Apgar scores were 9 and 10, respectively. The diagnosis of FVII deficiency may be difficult in the neonatal period because FVII levels are physiologically low in newborns [8]. The newborn in the present case had no bleeding signs, and therefore a FVII assay was not executed. In postoperative management, Ciprofloxacin (200 mg per 12 hours) was administered intravenously as a preventive antibiotic. Cefmetazole was excluded due to the possibility of vitamin K deficiency leading to hypoprothrombinemia and bleeding tendency. Tranexamic acid and vitamin K were administered for two days intravenously (1 KU per 8 hours) and subcutaneously (10 mg per 12 hours), respectively. Intramuscular injections were restricted to prevent intramuscular hematoma formation. There was no excessive vaginal bleeding or thrombotic signs. Stitch out was performed five days after the cesarean section, and the patient was discharged without unusual complications. At three weeks postoperative, she had swelling in both upper arms at the vitamin K injection site, lesions that were symmetric erythematous petechiae with itching and febrile senses (Fig. 2). She was referred to a dermatologist, and the lesions were diagnosed as a drug reaction. After drug treatment with outpatient follow-up, the lesions rapidly improved and disappeared.
FVII deficiency is characterized by wide molecular and clinical heterogeneity. The gene encoding FVII, F7, is located on chromosome 13 (13q34) and consists of nine exons and eight introns over a 12.8 kb genomic region. More than 250 mutations of F7 have been reported and missense mutations being the major type [9]. In congenital FVII deficiency patients, FVII levels are usually less than 10% of the normal range in homozygous or double heterozygous carriers and approximately 20% to 60% in heterozygous carriers. FVII levels usually increase in normal pregnancy, but no increase is seen in homozygous cases and only a moderate increase occurs in heterozygous individuals. Common presenting symptoms are bleeding into joints and muscles, excessive bruising, menorrhagia and epistaxis. The clinical severity of this disorder varies from life-menacing to symptomless [4]. However, compared to hemophilia A and B, there is a poor correlation between the FVII level and the severity of the bleeding [10]. This feature makes it difficult to predict which patients may need prophylactic replacement therapy prior to hemostatic challenges. The decision about such therapy should be based on previous bleeding episodes, FVII levels in a prenatal test, and the mode of delivery [11]. In spite of the poor correlation, the most serious bleeding is linked with low FVII activity levels and surgery [10]. In our case, the FVII activity of the patient was decreased (7.00%) in the prenatal test. The patient also had a history of successful bleeding control by preventive use of FFP preceding a dental extraction. From our patient's medical history, we decided to use only FFP and antifibrinolytic agents for surgical bleeding prevention. FFP is generally applied in most developing countries to achieve adequate hemostasis and there have been good results in mild cases. However, its application is related with several side effects, such as transmission of blood-borne viruses and blood volume overload. Recombinant activated FVII (rFVIIa, NovoSeven, Novo-Nordisk, Bagsvaerd, Denmark) has many advantages compared to blood products. These include more efficient FVII level elevation and no risk of blood-borne virus transmission. In postpartum hemorrhage, common non-operative management involves the use of uterine stimulants, bimanual compression of the uterus and uterine artery embolization [12]. More recently, rFVIIa has also been used to prevent or control bleeding in postpartum hemorrhage [13]. However, it has thrombotic risks, and there are no definitive target FVII levels or optimal treatment regimens to maintain hemostasis [14]. rFVIIa also has insufficient clinical data in the obstetric field, so its application is still limited. Antifibrinolytic agents are useful as adjunctive therapy in bleeding disorders. Tranexamic acid competitively restricts the activation of plasminogen to plasmin and improves clot stability. It is useful in bleeding control that occurs from mucosal surfaces and skin (oral bleeding, epistaxis, and menorrhagia) [15]. Vitamin K can be administered in cases of secondary hypoprothrombinemia that occurs due to vitamin K absorption or a synthesis problem. Although our patient seemed to have congenital FVII deficiency, we used vitamin K for prevention because a definitive diagnosis from genotyping was never obtained.
In conclusion, FVII deficiency can make excessive bleeding in surgical interventions, such as postpartum hemorrhage in cesarean sections. Clinicians need to ascertain the expected bleeding risk based on previous bleeding episodes and FVII levels. Replacement blood product therapy is a convenient process and mostly effective in patients with mild bleeding disorders. However, it has some adverse outcomes and rFVIIa can be more effective in elevating FVII levels and controlling bleeding in severe cases. Further study and evaluation are required on rFVIIa application in the obstetric field, and the use of rFVIIa should be considered in severe cases. In addition, careful examination of thrombotic signs and symptoms are required in the outpatient follow-up, as well as during the hospitalization period.
Figures and Tables
Acknowledgments
Thanks to all members of the Department of Obstetrics and Gynecology, Gangneung Asan Hospital, University of Ulsan College of Medicine.
References
1. Herrmann FH, Wulff K, Auerswald G, Schulman S, Astermark J, Batorova A, et al. Factor VII deficiency: clinical manifestation of 717 subjects from Europe and Latin America with mutations in the factor 7 gene. Haemophilia. 2009; 15:267–280.
2. Osterud B. Factor VII and haemostasis. Blood Coagul Fibrinolysis. 1990; 1:175–181.
3. Korea Hemophilia Foundation. Annual report 2010. Seoul: Korea Hemophilia Foundation;2011.
4. Mariani G, Herrmann FH, Dolce A, Batorova A, Etro D, Peyvandi F, et al. Clinical phenotypes and factor VII genotype in congenital factor VII deficiency. Thromb Haemost. 2005; 93:481–487.
5. Benlakhal F, Mura T, Schved JF, Giansily-Blaizot M. A retrospective analysis of 157 surgical procedures performed without replacement therapy in 83 unrelated factor VII-deficient patients. J Thromb Haemost. 2011; 9:1149–1156.
6. Giansily-Blaizot M, Biron-Andreani C, Aguilar-Martinez P, de Moeloose P, Briquel ME, Goudemand J, et al. Inherited factor VII deficiency and surgery: clinical data are the best criteria to predict the risk of bleeding. Br J Haematol. 2002; 117:172–175.
7. Kim BJ, Lee CH, Kim YH, Cho JS, Park YW. A case of vaginal delivery in woman with congenital factor V deficiency. Korean J Obstet Gynecol. 2002; 45:873–877.
8. Pike GN, Bolton-Maggs PH. Factor deficiencies in pregnancy. Hematol Oncol Clin North Am. 2011; 25:359–378.
9. Kwon MJ, Yoo KY, Lee KO, Kim SH, Kim HJ. Recurrent mutations and genotype-phenotype correlations in hereditary factor VII deficiency in Korea. Blood Coagul Fibrinolysis. 2011; 22:102–105.
10. Brummel Ziedins K, Rivard GE, Pouliot RL, Butenas S, Gissel M, Parhami-Seren B, et al. Factor VIIa replacement therapy in factor VII deficiency. J Thromb Haemost. 2004; 2:1735–1744.
11. Baumann Kreuziger LM, Morton CT, Reding MT. Is prophylaxis required for delivery in women with factor VII deficiency? Haemophilia. 2013; 19:827–832.
12. Kim TH, Lee HH, Kim JM, Ryu AL, Chung SH, Seok Lee W. Uterine artery embolization for primary postpartum hemorrhage. Iran J Reprod Med. 2013; 11:511–518.
13. Kobayashi T, Nakabayashi M, Yoshioka A, Maeda M, Ikenoue T. Recombinant activated factor VII (rFVIIa/NovoSeven(R)) in the management of severe postpartum haemorrhage: initial report of a multicentre case series in Japan. Int J Hematol. 2012; 95:57–63.
14. Lapecorella M, Mariani G. Factor VII deficiency: defining the clinical picture and optimizing therapeutic options. Haemophilia. 2008; 14:1170–1175.
15. Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013; 19:e1–e47.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download