Abstract
Objective
In this study, we evaluated the expression of FAS-associated factor 1 (FAF1) and heat shock protein 70 (HSP70) in normal ovary and ovarian cancer, and also analyzed the correlation between FAF1 and HSP70 in ovarian cancer.
Methods
The patient group consisted of 29 unrelated Korean women diagnosed as ovarian cancers and control samples were obtained from 7 patients who underwent oophorectomy for benign disease of uterus, and normal ovary was confirmed histologically from biopsy. We examined FAF1 and HSP70 expression by western blot analysis and immunohistochemical staining in normal ovary and ovarian cancer. Furthermore, we examined a correlation between FAF1 and HSP70 in ovarian cancer.
Results
The expression of FAF1 was lower in ovarian cancer than that in normal ovary (P=0.02), and the expression of HSP70 was increased in ovarian cancer in comparison to that in normal ovary (P=0.03). The expression of FAF1 was decreased in advanced stages (stage III or stage IV) as compared with early stages (stage I or stage II) (P=0.01). The expression of HSP70 was not significantly related with ovarian cancer histology (P=0.10), but the expression of HSP70 was most increased with papillary serous carcinomas and undifferentiated ovarian cancer. The expression of FAF1 was inversely correlated with the expression of HSP70 in ovarian cancer (Spearman correlation coefficience=-0.47).
Conclusion
We concluded that the expression of FAF1 or HSP70 each seems to have a meaning as a biomarker for early detection of ovarian cancer. The expressions of FAF1 and HSP70 seem to be more valuable in predicting ovarian cancer when used together because of their inverse correlation. This is the first study about the expression of FAF1 in ovarian cancer and the correlation between FAF1 and HSP70 expression in ovarian cancer.
Ovarian cancer is the fourth most common cause of cancer death in women and is the leading cause of death among gynecologic cancer in the United States, with an estimated 21,880 new cases and 13,850 deaths predicted in 2010 [1]. It is the second most common, but the most lethal gynecologic cancer in South Korea [2]. Since adequate screening methodologies are lacking, most women first present with either stage III or IV disease. Sixty-seven percent of patients are diagnosed at stage III and IV, with resultant low relative-survival rates [1]. Stage I disease is associated with a 90 percent cure rate, making early detection methods imperative [3]. Because detection and removal of tumors when still confined to the ovary confer substantial improvement in survival, recent research has focused on developing a blood-based biomarker screen to be used either alone or in combination with transvaginal sonography [4].
CA-125 is the most reliable serum marker for ovarian carcinoma [5]. Whereas its role in the screening of the malignancy is controversial [6]. Elevated serum CA-125 levels (>35 U/mL) can be detected in approximately 50% of patients with stage I and in more than 90% of those with advanced disease [5]. Mucinous tumors express this antigen less frequently than nonmucinous ones [7]. Tumor-associated antigens other than CA-125, such as CA-19-9, CA-15-3 and TAG 72, firstly identified in gastro-intestinal or breast malignancies, have been detected also in tissue and serum samples from patients with ovarian carcinoma [6,7]. In particular CA-19-9 offers the advantage of high sensitivity for mucinous histotype, which often fails to express CA-125 [7]. But, the roles of CA-125, CA-19-9 and currently used other markers in early detection of ovarian cancer are still not fully established [8]. Early detection of ovarian cancer remains challenging until now.
FAS is a member of the TNF receptor super-family that interacts with FAS ligand to mediate programmed cell death or apoptosis in a number of tissues. The interaction of FAS with its ligand allows the formation of a death-inducing signaling complex [9]. FAS-associated factor 1 (FAF1) was originally identified as an interactor of FAS that enhances apoptosis initiated through FAS and FAS ligand interaction [10]. FAF1 is a ~74-kDa protein composed of 650 amino acids, the amino terminal domain of which can bind to the cytoplasmic domain of FAS. Moreover, an ubiquitin-like domain localized to the carboxyl terminal region of FAF1 is required for proapoptotic activity [11]. FAF1 plays an important role in normal development and neuronal cell survival, whereas FAF1 downregulation may contribute to multiple aspects of tumorigenesis [12]. FAF1 is a tumor suppressor involves in regulation of apoptosis and NF-κB activity, as well as in ubiquitination and proteasomal degradation [12]. FAF1 expression was found to be down regulated in high percentage of human gastric carcinoma [13].
Heat shock protein (HSP) is first recognized after thermal stress, whereas de novo protein synthesis is generally reduced. In addition to heat, a variety of non-physiological events, including nutritional deprivation, physical (i.e., UV light, gamma-irradiation), or chemical stressors (i.e., heavy metals, oxidative stress, cytostatic drugs, amino acid analogues, anti-inflammatory drugs, and alkyl-lysophospholipids) have been identified as potent inducers of a classical heat shock (stress) protein response. HSP70 participates in the folding of newly synthesized proteins, translocation of intracellular proteins, assembly and disassembly of oligomeric proteins, and in controlling the activity of regulatory proteins [14,15]. As HSP70 exerts its various roles through binding to various chaperone cofactors or co-chaperones, the fate of substrate proteins is determined by the nature of the co-chaperones [15]. Elevated HSP70 plays a key role in protecting malignant cells from spontaneous apoptosis, but exact mechanisms are not yet determined. HSP70 is evaluated as a marker of early hepatocellular carcinoma [16].
More recently FAF1 was found as a new HSP70-binding protein employing immunoprecipitation and identification of the bound proteins using matrix-associated laser desorption and ionization time-of flight mass spectrometry [17]. Interaction mapping indicated that the 82-180 sequence of FAF1 directly binds to the N-terminal region containing sequence 1-120 of HSP70. This binding is very tight regardless of ATP and heat shock treatment [17]. FAF1 regulates the chaperone activity through binding to chaperones in the heat shock-mediated signaling pathway because FAF1 contains two ubiquitin-like domains and one ubiquitin-like module. FAF1 inhibits the HSP70 chaperone activity of refolding denatured protein substrates and accelerated heat shock-induced cell death in a binding dependent manner [17]. Transient overexpression of FAF1 inhibits chaperone activity of HSP70, suggesting that FAF1 is possibly a novel co-chaperone of HSP70 [17].
This study was undertaken to evaluate the expression of HSP70 and FAF1 in normal ovary and ovarian cancer. We also analyzed the correlation between HSP70 and FAF1 expression in ovarian cancer. Therefore, the purpose of this study was investigate whether we can use the expression of HSP70 and FAF1 as a biomarker for early detection of ovarian cancer.
The study subjects were recruited between September 2000 and December 2005 at the Department of Obstetrics and Gynecology of Ewha Womans University Hospital. The patients with ovarian cancers had undergone total hysterectomy and bilateral salphingo-oophorectomy, and the disease was confirmed histologically from biopsies. This study was limited to a native Korean population that presented to a single hospital for treatment. The patient group consisted of 29 unrelated Korean women diagnosed as ovarian cancers (stage I, II, III, and IV) according to FIGO (International Federation of Gynecology and Obstetrics) staging for Primary Carcinoma of the Ovary. Control samples were obtained from 7 patients who underwent oophorectomy for benign disease of uterus, and normal ovary was confirmed histologically from biopsy. Patients with benign ovarian and paratubal cysts were excluded from the control group. The parameters of age, recurrence, prognostic profile and others were reviewed from medical records.
Of the 29 patients, Stage I or II ovarian cancers were present in 7 patients, and stage III or IV in 22 patients (Tables 1, 2). That is, most women first present with either stage III or IV disease. Among those, there were 11 papillary serous carcinoma patients, 3 endometrioid adenocarcinoma patients, and 4 clear cell carcinoma patients (Tables 1, 2). Serum CA-125 level was above normal range (higher than cutoff point 35 U/mL) in 24 patients, and serum CA-19-9 level was above normal range (higher than cutoff point 37 U/mL) in 7 patients (Tables 1, 2).
Tissues were washed twice with phosphate buffered saline and then suspended in an total protein extraction kit (Intron Biotechnology, Seongnam,, Korea) on ice for 15 minutes. Lysates were cleared by centrifugation at 13,000 rpm for 20 minutes. The protein concentrations of the lysates were determined by the bicinchoninic acid protein assay system (Pierce, Rockford, IL, USA), and equal protein amounts of each sample were separated by electrophoresis on polyarcylamide gels. Equal protein loading was confirmed by Coomassie blue staining of duplicate gels after electrophoresis. Proteins were transferred to a polyvinylidene difluoride membrane (Invitrogen, Carlsbad, CA, USA) by electrotransfer. The membrane was pre-incubated for 2 hours in Tris buffered saline (TBS) containing 5% skim milk and 0.05% Tween 20 (TBS-T). The membrane was incubated for 1 hour at room temperature in TBS-T plus antibodies. Mouse polyclonal anti-FAF1 antibody was obtained from Abnova (1:1,000 dilution; Heidelberg, Germany). Mouse monoclonal anti-HSP70 antibody (clone C92F3A-5) was obtained from Enzo (1:1,000 dilution; Plymouth Meeting, PA, USA). Mouse monoclonal anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) antibody was obtained from Abcam (1:1,000 dilution; Cambridge, UK). After incubation, the membranes were washed five times with TBS-T and then incubated with the biotin-conjugated secondary antibody (Vectastain Elite Kit, Vector Laboratories, Burlingame, CA, USA) for 45 minutes at room temperature. GAPDH, a housekeeping protein, was used to normalize the loading and hence allow semi-quantification of FAF1 and HSP70 proteins. Light microscopy and analysis 3.1 (Soft Imaging System, Münster, Germany), an image processing and analysis program, were used for evaluating FAF1 and HSP70 expression.
Serial 3-mm sections were taken from formalin-fixed, paraffin-embedded tissue blocks and used for immunohistochemistry. Briefly, slides were deparaffinized twice in xylene for 5 minutes and rehydrated through graded ethanol solutions to distilled water. Antigen retrieval was performed by heating sections in a microwave oven in 10 mM citrate buffer (pH 6.0). Sections were then immunostained using an automated machine (Bond Automated Immunohistochemistry, Leica, Wetzlar, Germany) and the bond polymer detection system with counterstain (Leica Autostainer XL, Wetzlar, Germany). The process included endogenous peroxidase blocking by 3% hydrogen peroxide for 5 minutes, and incubation with primary antibodies at room temperature for 30 minutes. Rabbit polyclonal anti-FAF1 antibody was obtained from Atlas (1:200 dilution; Stockholm, Sweden) and mouse monoclonal anti-HSP70 antibody (clone C92F3A-5) was obtaianed from Enzo (1:2,000 dilution). Primary antibodies were detected using polymeric horseradish peroxidase-linked antibody conjugates as secondary antibody, and color reaction was expressed using 3,3'-diaminobenzidine. The normal tissue and ovarian cancer region were defined as the region of interest (ROI) and HSP70, FAF1 -positive staining was analyzed. When 20% or more of cells in a given specimen were positively stained, the sample was graded as HSP70, FAF1 positive; it was graded negative when fewer than 20% of cells were stained.
Analyses were performed using SAS ver. 9.2 (SAS Institute, Cary, NC, USA). P-values were two-sided and considered to be valuable when they were less than 0.05. The statistical significance of the data was analyzed using Wilcoxon rank sum test, Kruskal-Wallis method. The correlation of expression of FAF1 and HSP70 in ovarian cancer was analyzed using Spearman rank test.
To study possible effect of FAF1 on ovarian cancer, we first assessed the expression of FAF1 in normal ovary and ovarian cancer (Table 3; Figs. 1, 2). The expression of FAF1 was lower in ovarian cancer than that in normal ovary in western blotting (2.03±2.43 vs. 4.23±2.47, P=0.02) (Table 3). But, by immunohistochemical staining, the FAF1 expression in ovarian cancer was not statistically different in contrast to that in normal ovary.
To study possible effect of HSP70 on ovarian cancer, we assessed the expression of HSP70 in normal ovary and ovarian cancer (Table 3; Figs. 1, 2). By western blotting, the expression of HSP70 in ovarian cancer was increased in comparison to that in normal ovary (0.83±0.57 vs. 0.38±0.24, P=0.03) (Table 3). By immunohistochemical staining, the expression of HSP70 in ovarian cancer was also increased in contrast to that in normal ovary.
The expression of FAF1 was decreased when serum CA-19-9 level was above the normal range (more than cutoff point 37 U/mL) in comparison with the normal range (less than 37 U/mL) (P=0.02). Therefore, the expression of FAF1 was related with a valuable CA-19-9 in ovarian cancer. Interestingly, the expression of FAF1 was decreased in advanced stages (stage III or stage IV) as compared with early stages (stage I or stage II) (P=0.01), thus its expression tended to decline with increasing stage. There was no relationship between the expression of FAF1 and other factors such as age, pathology, lymph node metastasis, recurrence, and level of serum CA-125 (Table 1).
The expression of HSP70 was increased when serum CA-19-9 level was above normal range in comparison with the normal range, with marginal statistical significance (P=0.08). Therefore, the expression of HSP70 was slightly related with a valuable CA-19-9 in ovarian cancer. Interestingly, the expression of HSP70 was not significantly related with ovarian cancer histology (P=0.10), but the expression of HSP70 was most increased with papillary serous carcinomas. The expression of HSP70 was not related with other factors such as age, stage, lymph node metastasis, recurrence, level of serum CA-125 (Table 2).
The correlation coefficience of FAF1 and HSP70 in ovarian cancer by Spearman rank test was -0.47 (Fig. 3). There was a moderately inverse correlation between the expression of FAF1 and that of HSP70 in ovarian cancer.
In our study, the expression of FAF1 was decreased in ovarian cancer than that in normal ovary, and the expression of HSP70 was increased in ovarian cancer in comparison to that in normal ovary. The expression of FAF1 was inversely correlated with the expression of HSP70. The expression of FAF1 or HSP70 each seems to have a meaning as a prognostic factor in ovarian cancer patients. Furthermore, the expressions of FAF1 and HSP70 seem to be more valuable as prognostic factors in ovarian cancer patients when used together because of their inverse correlation. The expression of FAF1 can be used as a predictive factor of ovarian cancer because that expression is related with the value of CA-19-9 tumor marker.
Recently, it was identified that loss of FAF1 was a common occurrence in some human cancers and implicated FAF1 as a tumor suppressor whose loss contributes to cancers of varying origins [12]. We identified that the expression of FAF1 was lower in ovarian cancer than that in normal ovary. Loss or down regulation of FAF1 has been observed in various human cancers including human gastric carcinoma [13]. Recurrent mono-allelic and homozygous deletion of FAF1 has been reported in mantle cell lymphoma [18], and single nucleotide polymorphism (SNP) testing revealed that FAF1 may be associated with a genetic locus implicated in susceptibility to Crohn's disease, an inflammatory bowel disorder that conveys an increased risk for colorectal cancer [19]. Moreover, genomic loss at D1S427-FAF1 (1p32.3) locus has been reported in microarray Chromosomal Comparative Genomic Hybridization of uterine cervix carcinomas [20]. Our study suggests the possibility that the expression of FAF1 can be exploited as a biomarker for early detection of ovarian cancer.
HSP70 acts as a chaperone molecule for antigenic peptides derived from tumor cells, leading to an antitumor immune recognition by cytotoxic T lymphocytes [21], which also induces TNF-α production [22], and has been found to protect tumor cells from TNF-mediated cytotoxicity [23]. In addition, HSP70 is induced in tumor cells to overcome the stressful conditions faced by the tumor, such as lack of nutrients, oxygen, or antitumor immune response contributing to their survival and acts an endogenous tumor promoter in vivo [24]. HSP70 inhibits a death pathway involving cell digestion by lysosome-derived cathepsins, because HSP70 has been shown to be enriched in the lysosomal membranes of tumor cells and depletion of this store of HSP70 leads to spontaneous death related to leakage of cathepsins into the bulk cytoplasm of diverse cancer cells [25]. Necrosis arising from inhibition of programmed cell death (PCD) by HSP70 overexpression results in the release of cell contents into the tumor milieu and the initiation of an inflammatory environment in the vicinity of the tumor that favors angiogenesis, tumor cell invasion and metastasis [26]. We found that the expression of HSP70 was increased in ovarian cancer in comparison to that in normal ovary. Upregulation of HSP70 has been observed in various human cancers including early hepatocellular carcinoma [16]. HSP70 is associated with carcinogenesis of the oral epithelium [27]. HSP70 is of significance as a tumor marker in osteosarcoma [28]. It is suggested that the expression of HSP70 can be used to a biomarker for early detection of ovarian cancer similar to that of FAF1.
The expression of HSP70 by immunohistochemical staining was shown to be same as that by western blot analysis. In other words, the expression of HSP70 in ovarian cancer was remarkably increased in contrast to that in normal ovary by immunohistochemical staining. However, the expression of FAF1 between in two groups was not different by immunohistochemical staining. We thought that the expression of FAF1 could not detected well by immunohistochemical staining with the selected primary antibody or detection kit. This study suggested the possibility that the expression of HSP70 could be exploited as a ovarian cancer screening biomarker, additionally.
Downregulation of FAF1 would thus be expected to promote the survival of a tumor cell as well as its resistance to the efforts of anti-cancer therapies [12]. Importantly, NF-κB and ubiquitin proteasomal pathways are both negatively regulated by overexpression of FAF1 [29], and both of these pathways are currently being targeted pharmacologically as a chemotherapeutic approach to treat various human cancers [30,31]. The NF-κB and ubiquitin proteasomal pathways have been targeted therapeutically in garstric [32] and uterine carcinomas [33] as well as in mantle cell lymphomas [34], all of which are cancers that exhibit frequent downregulation of FAF1 [13,18,20]. Thus, loss of FAF1 in cancer cells, leading to aberrant NF-κB and proteasomal signaling, could be exploited as a potential biomarker for responsiveness of these tumors to these types of pathway inhibitors [12]. This is the first study about the expression of FAF1 in ovarian cancer. When we analyzed FAF1 expression with the clinicopathologic finding of ovarian cancer patients, we found that the expression of FAF1 was related with a valuable CA-19-9 in ovarian cancer. Interestingly, the expression of FAF1 tended to decline according to increasing stages. In these findings, we noticed that the expression of FAF1 had a possibility as a tumor marker in ovarian cancer, and could be used to predict the ovarian cancer progression. Additionally, it is possible that the expression of FAF1 can be used as a factor in the treatment of ovarian cancer to determine the response to treatment.
HSP70 is evaluated as a marker of early hepatocellular carcinoma [16]. HSP70 has been involved not only with poor tumor differentiation in breast, ovary and oral cancer but also with increased cell proliferation in breast, uterine cervix, and lung cancer. It also has been involved in lymph node metastasis of breast and colon cancer, increased tumor size of uterine cervix, and higher clinical stage in colon and oral cancer [27,35,36,37,38,39]. We also found that the expression of HSP70 was slightly related with a valuable CA-19-9 in ovarian cancer, especially, among clinicopathologic factors. As well, the expression of HSP70 was not significantly related with ovarian cancer histology, but the expression of HSP70 was most increased with papillary serous carcinomas. HSP70 was reported to be associated with poor differentiation of the ovarian cancer in other study [36]. It means that an increase in the expression of HSP70 may be related with the poor differentiation of ovarian cancer similar as breast and oral cancer, and the expression of HSP70 has a possibility as a prognostic factor in ovarian cancer patients. We can also suggest the possibility that HSP70 can be used as a factor in the treatment of ovarian cancer to determine the response to treatment.
We found that the expression of FAF1 and HSP70 had a moderately inverse correlation in ovarian cancer. This is the first study about the correlation between FAF1 and HSP70 expression in ovarian cancer even though we did not search the accurate action mechanism of two proteins in ovarian cancer in this study. It has been identified that the 82-180 amino acid region of FAF1 directly interacts with the N terminus (amino acids 1-120) of the ATPase domain of HSP70, and that FAF1 acts as modulator of HSP70 function [17]. The overexpression of FAF1 renders cell more sensitive to heat shock, probably due to inhibition of HSP70 chaperone activity such as refolding denatured protein substrates, accelerated heat shock-induced SAPK/JNK activation, and raised heat shock-induced cell death in a binding dependent manner [17,29]. Further studies of the interaction between FAS and HSP70 are needed to explain this discrepancy, but FAF1 plays a role in the regulation of stress-induced cell death using HSP70 as a binding partner. Thus, we noticed that both expressions was related with each other in ovarian cancer, and then the expressions of FAF1 and HSP70 are more valuable in predicting ovarian cancer when used together.
In summary, we evaluated that the expression of FAF1 was decreased, and that of HSP70 was increased in ovarian cancer in contrast to normal ovary and both expressions had a moderate inverse correlation in ovarian cancer. The expression of FAF1 or HSP70 each seems to have a meaning as a prognostic factor in ovarian cancer patients. Furthermore, the expression of FAF1 and HSP70 seem to be more valuable as prognostic factors in ovarian cancer patients when used together because of their inverse correlation. Additionally the expressions of FAF1 and HSP70 are related with a valuable CA-19-9 in ovarian cancer. It means that we can use those as a tumor marker to determine the response to treatment. It is further needed to study the role of FAF1 and HSP70 as predictive markers in ovarian cancer and mutual action mechanism of those in ovarian cancer.
Figures and Tables
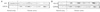 | Fig. 1The western blot analysis of FAS-associated factor 1 (FAF1) and heat shock protein 70 (HSP70) with GAPDH expression in normal ovary and ovarian cancer. The FAF1 expression in ovarian cancer was lower than that in normal ovary (A). The HSP70 expression in ovarian cancer was higher than that in normal ovary (B). |
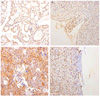 | Fig. 2The immunohistochemical staining of FAS-associated factor 1 (FAF1) and heat shock protein 70 (HSP70) expression in normal ovary and ovarian cancer. The FAF1 expression in ovarian cancer (A, ×200) was not different in contrast to that in normal ovary (B, ×100). The HSP70 expression in ovarian cancer (C, ×200) was increased in contrast to that in normal ovary (D, ×200). |
 | Fig. 3The correlation graph of expression of FAS-associated factor 1 (FAF1) and heat shock protein 70 (HSP70) in ovarian cancer. The correlation coefficience of FAF1 and HSP70 in ovarian cancer by Spearman rank test was -0.47. There was a moderately inverse correlation between the expression of FAF1 and that of HSP70 in ovarian cancer. |
Table 1
The correlation of clinicopathologic finding with the expression of FAS-associated factor 1 by western blotting analysis in ovarian cancer

References
1. American Cancer Society. Cancer facts & figures. Atlanta: American Cancer Society;2010.
2. National Cancer Control Institute. 2008 National cancer statistics. Goyang: National Cancer Center;2010.
3. Ren J, Cai H, Li Y, Zhang X, Liu Z, Wang JS, et al. Tumor markers for early detection of ovarian cancer. Expert Rev Mol Diagn. 2010; 10:787–798.
4. Kabawat SE, Bast RC Jr, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol. 1983; 2:275–285.
5. Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989; 4:1–12.
6. Berek JS, Bast RC Jr. Ovarian cancer screening. The use of serial complementary tumor markers to improve sensitivity and specificity for early detection. Cancer. 1995; 76:10 Suppl. 2092–2096.
7. Gadducci A, Ferdeghini M, Prontera C, Moretti L, Mariani G, Bianchi R, et al. The concomitant determination of different tumor markers in patients with epithelial ovarian cancer and benign ovarian masses: relevance for differential diagnosis. Gynecol Oncol. 1992; 44:147–154.
8. Anderson GL. Ovarian cancer biomarker screening: still too early to tell. . Womens Health (Lond Engl). 2010; 6:487–490.
9. Yu JW, Shi Y. FLIP and the death effector domain family. Oncogene. 2008; 27:6216–6227.
10. Chu K, Niu X, Williams LT. A Fas-associated protein factor, FAF1, potentiates Fas-mediated apoptosis. Proc Natl Acad Sci U S A. 1995; 92:11894–11898.
11. Ryu SW, Kim E. Apoptosis induced by human Fas-associated factor 1, hFAF1, requires its ubiquitin homologous domain, but not the Fas-binding domain. Biochem Biophys Res Commun. 2001; 286:1027–1032.
12. Menges CW, Altomare DA, Testa JR. FAS-associated factor 1 (FAF1): diverse functions and implications for oncogenesis. Cell Cycle. 2009; 8:2528–2534.
13. Bjorling-Poulsen M, Seitz G, Guerra B, Issinger OG. The pro-apoptotic FAS-associated factor 1 is specifically reduced in human gastric carcinomas. Int J Oncol. 2003; 23:1015–1023.
14. Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996; 381:571–579.
15. Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998; 92:351–366.
16. Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, et al. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003; 37:198–207.
17. Kim HJ, Song EJ, Lee YS, Kim E, Lee KJ. Human Fas-associated factor 1 interacts with heat shock protein 70 and negatively regulates chaperone activity. J Biol Chem. 2005; 280:8125–8133.
18. Bea S, Salaverria I, Armengol L, Pinyol M, Fernandez V, Hartmann EM, et al. Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood. 2009; 113:3059–3069.
19. Weersma RK, Stokkers PC, Cleynen I, Wolfkamp SC, Henckaerts L, Schreiber S, et al. Confirmation of multiple Crohn's disease susceptibility loci in a large Dutch-Belgian cohort. Am J Gastroenterol. 2009; 104:630–638.
20. Hidalgo A, Baudis M, Petersen I, Arreola H, Pina P, Vazquez-Ortiz G, et al. Microarray comparative genomic hybridization detection of chromosomal imbalances in uterine cervix carcinoma. BMC Cancer. 2005; 5:77.
21. Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995; 269:1585–1588.
22. Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000; 6:435–442.
23. Jaattela M. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J Immunol. 1993; 151:4286–4294.
24. Soti C, Csermely P. Molecular chaperones in the etiology and therapy of cancer. Pathol Oncol Res. 1998; 4:316–321.
25. Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Hoyer-Hansen M, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004; 200:425–435.
26. Proskuryakov SY, Konoplyannikov AG, Gabai VL. Necrosis: a specific form of programmed cell death? Exp Cell Res. 2003; 283:1–16.
27. Kaur J, Das SN, Srivastava A, Ralhan R. Cell surface expression of 70 kDa heat shock protein in human oral dysplasia and squamous cell carcinoma: correlation with clinicopathological features. Oral Oncol. 1998; 34:93–98.
28. Trieb K, Thurnher D, Bakroeva M, Kotz R, Kornfehl J. Reversible downregulation of telomerase activity by hyperthermia in osteosarcoma cells. Int J Hyperthermia. 2000; 16:445–448.
29. Park MY, Jang HD, Lee SY, Lee KJ, Kim E. Fas-associated factor-1 inhibits nuclear factor-kappaB (NF-kappaB) activity by interfering with nuclear translocation of the RelA (p65) subunit of NF-kappaB. J Biol Chem. 2004; 279:2544–2549.
30. McConkey DJ, Zhu K. Mechanisms of proteasome inhibitor action and resistance in cancer. Drug Resist Updat. 2008; 11:164–179.
31. Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009; 8:33–40.
32. Fan XM, Wong BC, Wang WP, Zhou XM, Cho CH, Yuen ST, et al. Inhibition of proteasome function induced apoptosis in gastric cancer. Int J Cancer. 2001; 93:481–488.
33. Birle DC, Hedley DW. Suppression of the hypoxia-inducible factor-1 response in cervical carcinoma xenografts by proteasome inhibitors. Cancer Res. 2007; 67:1735–1743.
34. Yang DT, Young KH, Kahl BS, Markovina S, Miyamoto S. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Mol Cancer. 2008; 7:40.
35. Lazaris AC, Chatzigianni EB, Panoussopoulos D, Tzimas GN, Davaris PS, Golematis BC. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res Treat. 1997; 43:43–51.
36. Athanassiadou P, Petrakakou E, Sakelariou V, Zerva C, Liossi A, Michalas S, et al. Expression of p53, bcl-2 and heat shock protein (hsp72) in malignant and benign ovarian tumours. Eur J Cancer Prev. 1998; 7:225–231.
37. Kim KK, Jang TJ, Kim JR. HSP70 and ER expression in cervical intraepithelial neoplasia and cervical cancer. J Korean Med Sci. 1998; 13:383–388.
38. Nanbu K, Konishi I, Mandai M, Kuroda H, Hamid AA, Komatsu T, et al. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect Prev. 1998; 22:549–555.
39. Bonay M, Soler P, Riquet M, Battesti JP, Hance AJ, Tazi A. Expression of heat shock proteins in human lung and lung cancers. Am J Respir Cell Mol Biol. 1994; 10:453–461.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download