Abstract
Objective
We sought to investigate the clinicopathologic features of ovarian squamous cell carcinomas arising from mature cystic teratomas (MCT) and to report our clinical experience and lessons learned.
Methods
From January 1993 to November 2012, a total of 6,260 women with ovarian MCT were surgically treated at Cheil General Hospital and Women's Healthcare Center. Among them, the cases with malignant transformation to squamous cell carcinoma were included in this analysis. Patient demographic characteristics, surgical findings, and prognosis were evaluated retrospectively.
Results
Of the 6,260 ovarian MCT patients, four (0.06%) had ovarian squamous cell carcinoma arising from MCT. The mean patient age was 43 years (range, 35-51 years), and the mean tumor size was 12 cm (range, 9-16 cm), with two patients in the International Federation of Gynecology and Obstetrics stage I and the other two in stage III. Upon preoperative imaging, all cases were expected to be benign ovarian tumors, but the preoperative squamous cell carcinoma antigen level was elevated from 1.5 ng/mL in stage Ia to 11.3 ng/mL in stage IIIc, suggesting malignancy, while the CA-125 level was normal in two of the three patients who received the test. Optimal debulking surgery was performed and adjuvant chemotherapy was used in all patients, but death from the recurrence of disease occurred in one patient, whose overall survival was 10 months.
Conclusion
Ovarian squamous cell carcinoma arising from MCT is extremely rare, and it is rarely diagnosed preoperatively on imaging workups. Measuring the squamous cell carcinoma antigen level might be a useful diagnostic clue, and it might also be predictive of the tumor stage. An adequate staging surgery should be included in the standard treatment, but multicenter studies are needed to confirm this.
Mature cystic teratomas (MCT) of the ovary, better known as dermoid cysts, are the most common ovarian tumors in both adolescents and reproductive-aged women. For the management of benign adnexal masses, the gold standard treatment is laparoscopic surgery [1], and one of the most common indications for laparoscopic adnexal surgery is due to the presence of ovarian MCT. As a histologic subclass of ovarian germ-cell tumors, MCTs account for more than 95% of all ovarian teratomas. Because they are almost exclusively benign [2], surgeons may be insensitive to the risk of malignancy and may be less aggressive in the extent of the surgical tumor resection. However, not all cases of MCT remain benign. Secondary malignant transformations have been observed in rare cases of MCT, with a reported incidence of 0.2% to 3% [3,4,5,6]. It is thought that the malignant transformation of MCTs can occur in many tissue components, but most MCTs are usually detected 15 to 20 years before they undergo the transformation, so there is little information available on this topic [7]. However, we do know that squamous cell carcinoma (SCC) arising from the ectoderm is the most common secondary neoplasm, comprising 80% of the malignant transformations, and this may hold true for MCT transformations as well, which could guide more specific therapies.
Since there is no definite symptom or sign, even in radiologic imaging, it is challenging to preoperatively diagnose ovarian SCC arising from MCT unless the tumors are advanced-stage. Thus, most cases reported to date seem to be diagnosed by postoperative histopathologic analysis. However, an unexpected diagnosis of tumor malignancy during surgery may interrupt performing complete surgical management at that time, which can adversely affect prognosis. Moreover, it is well recognized that SCC-MCT has a poor prognosis, and no standard treatment is available because of its rarity [8]. Therefore, we aimed to investigate the clinicopathologic characteristics of SCC-MCT and to report our clinical experience and lessons learned in order to offer information that may remedy the lack of standardized treatment options.
Cases of both MCT and SCC rising from MCT that were pathologically diagnosed at Cheil General Hospital and Women's Healthcare Center from January 1993 to November 2012 were retrospectively identified using the disease coding system from the institutional electronic database. In Korea, the International Classification of Disease, 9th revision, Clinical Modification (ICD-9-CM) coding system is used to classify diagnoses and procedures. The codes of the ICD-9-CM were extracted and used to calculate the rate of SCC-MCT among all cases of MCT. In cases of confirmed SCC-MCT, the medical records were thoroughly reviewed.
Based on the institutional policy of Cheil General Hospital and Women's Healthcare Center, routine examinations for evaluating adnexal masses do not include serum tumor markers or radiologic imaging like computed tomography (CT) or magnetic resonance imaging (MRI), but it is acceptable to perform these tests according to the clinician's suspicion in order to exclude the possibility of malignancy. Serum tumor markers were considered normal when the SCC antigen (SCC-Ag) was <0.5 ng/mL and the CA-125 level was <35 U/mL. Three experienced pathologists reviewed each tissue sample obtained during surgery, and the slides were re-confirmed for the present study. Tumor stage was determined according to the International Federation of Gynecology and Obstetrics (FIGO) surgical staging system.
In general, after surgical management of ovarian cancer, serum CA-125 levels were routinely measured before every cycle of chemotherapy. CT or positron emission tomography/computerized tomography (PET/CT) imaging was obtained after every three cycles of chemotherapy to track disease progression. After completing the initial treatment, routine follow-ups comprised of a clinical examination and CA-125 level measurements were performed every three months for the next two years and every three to six months for the subsequent three years.
Patient demographic characteristics such as age, menopause status, obstetric history, and preoperative serum tumor markers were evaluated, as well as surgicopathologic findings such as operative findings, surgical procedures, and histopathologic reports. Clinical course information such as disease recurrence and adjuvant chemotherapy was also evaluated. Disease free survival was calculated as the time in months from the date of diagnosis to either the date of recurrence or the date of last follow-up. Overall survival was calculated as the time in months from the date of diagnosis to either the date of death or the date of last follow-up.
During the study period, 6,260 patients underwent surgery for ovarian MCT at Cheil General Hospital and Women's Healthcare Center, and of them, four (0.06%) patients were confirmed to have SCC arising from MCT. The mean patient age was 43.3 years (range, 35-51 years), and all patients were premenopausal (Table 1). Initially, all cases were misdiagnosed as ovarian benign teratomas on ultrasound, with or without CT, except for one case, which was suspected as MCT with malignant transformation on CT (Fig. 1). Two patients presented with abdominal pain and the other two had no symptoms. In the asymptomatic patients, the tumors were incidentally detected on routine examination.
To evaluate serological markers of malignancy, both CA-125 and SCC-Ag levels were measured in all patients, although one patient's (patient 3) initial CA-125 measurement was lost due to a long period of follow-up. Of interest, the preoperative SCC-Ag levels were elevated in all patients, and the levels increased in accordance with FIGO stage severity while the CA-125 levels were normal in the two patients with stage I tumors.
In these patients with ovarian SCC-MCT, contralateral ovaries showed MCT in three cases, but the MCTs showed no evidence of malignant transformation (Table 2). The mean tumor size of SCC-MCT was 11.8 cm (range, 9-16 cm) (Fig. 2), and frozen section diagnosis was performed in only two patients. In patient four, severe malignant-looking features such as periovarian adhesion and ascites were present, and since this was considered inflammatory change, laparoscopic cystectomy of the ovarian tumor and adhesiolysis was performed. All patients underwent surgical staging including total hysterectomy, bilateral salpingo-oophorectomy, and lymphadenectomy, although omentectomy was omitted in a patient because of a scarce amount of omentum. Two patients (50%) had stage I tumors and the other two patients had stage III tumors. Of the stage III patients, one was stage IIIa with microscopic omental and abdominal peritoneal tumors, and one was stage IIIc with a 3 cm-omental tumor and paraaortic nodal invasion.
All patients received adjuvant chemotherapy, even the patient with stage Ia disease, as per the clinician's decision. The first-line chemotherapeutic agent was either BOMP (bleomycin/vincristine/mitomycin C/cisplatin) or platinum based-paclitaxel (Table 3). Recurrence occurred in one patient with the most advanced stage (IIIc). In this patient, both of the serum tumor makers, CA-125 and SCC-Ag, had decreased during the three-cycle chemotherapy regimen, but PET/CT scans showed new lesions in liver and paraaortic lymph nodes. She died from the rapidly progressed disease despite the adjustments made to her chemotherapeutic regimen. The other three patients have been well and free from disease for over seven years since their first surgery and adjuvant chemotherapy. Mean OS was 79 months (range, 10-131 months).
Ovarian SCC-MCT is not a well-known disease due to its extreme rarity. In fact, in this study, 6,260 cases of MCT spanning 20 years were retrospectively analyzed, and only four patients were diagnosed with SCC-MCT. The disease incidence in our population was 0.06%, which is much lower than that of other studies [3,4,5,6], but identifying the exact population incidence is difficult and unclear due to the rarity of this condition. There are some explanations for such a low incidence in our study population. Firstly, since the Cheil General Hospital and Women's Healthcare Center is a secondary medical center, patients that had a high suspicion of malignancy might have been lost to follow-up before the surgery. In addition, the present study included cases from a 20-year period, and the possibility of misreading the pathology as a false negative is feasible, especially pathology from the early half of the study period.
In order to distinguish between MCT and MCT that has transformed to SCC, identifying clinical signs and differences between these stages is essential. The majority of ovarian SCC-MCTs are detected in postmenopausal women in their fifties and sixties, while MCT is common in younger women in their teens and twenties [9]. In the present study, the mean age of the patients was 43.3 years, and all of them were premenopausal, which may be due to early detection secondary to general use of ultrasonography in young women. Along with patient age, the size of the tumor is proposed to be another clinical characteristic that differs between SCC-MCT and MCT, with several reports indicating that SCC-MCT tumors are larger than benign teratomas [10]. In the largest study of SCC-MCT patients, Kikkawa et al. [10] reported that the mean tumor size among 37 cases of SCC-MCTs and 92 cases of MCTs was 152 and 88 mm, respectively (P<0.0001). The optimal cutoff values for malignancy screening were based on receiver-oriented curve analyses, and these cutoffs were determined to be 45 years of age and a tumor size of 99 mm. In another retrospective study by Kim et al. [8], eight cases of ovarian SCC-MCT were studied, and the mean tumor size was 13.3 cm, which was similar to the 11.8 cm tumor size in our study. According to these tumor size and patient age findings, using the screening criteria suggested by Kikkawa et al. [10] may be a reasonable approach to identifying SCC-MCT.
Another potential tool to detect malignant transformation of MCTs is the use of serum markers. One recent review article analyzed 277 cases of SCC-MCTs from 64 published studies [7]. In the report, 48 of 52 patients (86.5%) had serum concentrations high in SCC-Ag, and 36 of 51 patients (71%) had high CA-125 levels. Although there were no significant correlations between the levels of both tumor markers and FIGO stage, higher levels of both markers were predictive of poor survival prognosis. On the other hand, Kang et al. [11] showed that serum SCC-Ag was elevated in four of seven patients (57%), but CA-125 was not elevated in any of the 11 patients who received the test. Likewise, all cases in the present study had high SCC-Ag levels, which showed gradual increases according to the FIGO stage, and the CA-125 level was elevated in only one advanced-stage case. Taken together, these results suggest that SCC-Ag level may be associated with tumor prognosis and can be a more useful diagnostic clue than CA-125 for ovarian SCC-MCTs. These serum makers are valuable since it is nearly impossible to obtain a preoperative diagnosis merely by physical examination and ultrasound without using expensive imaging techniques such as MRI or CT scans [12]. A few reports have addressed MRI and CT findings in which malignancy is suspected. In these imaging studies, the presence of a nodular-forming and soft tissue-enhancing component, an obtuse angle between the soft tissue and the inner wall of the cyst, and extracapsular tumor growth with extension into adjacent structures or metastasis is highly predictive of malignancy [13]. However, an accurate diagnosis is still challenging, and a preoperative diagnosis was made for only one case in our study.
The prognosis of ovarian SCC-MCT varies according to the FIGO stage, and therefore, identifying the correct tumor stage is important. One review article reported that two-year disease-free survival was 100% for patients with stage I and II tumors, 30% for stage III tumors, and 0% for stage IV tumors [14]. Another report showed that five-year survival rates for adequately staged patients were 95% for stage I, 80% for stage II, and 0% for patients with stage III and IV disease [10].
To determine the exact stage of the tumor, and therefore, the appropriate adjuvant therapy, complete staging surgery including lymphadenectomy is crucial. Moreover, a literature review suggested that lymphadenectomy improved survival in patients with advanced-stage disease (59.2 months of mean OS in lymphadenectomy group vs. 40.4 months of mean OS in no lymphadenectomy group), despite only 14% (23 of 164) of these cases undergoing an adequate staging surgery [7]. In our study, all four patients underwent staging surgery, which included lymph node dissection as well as a long-term follow-up, and consequently, a case of recurrence was detected. These results imply that an initial aggressive surgery with complete staging can be helpful for better survival outcome. In adjuvant chemotherapy, the best regimens for ovarian SCC-MCT have not been determined through clinical trials, but some researchers reported that the treatment of this disease is similar to that of epithelial ovarian cancer according to the published data [7]. Therefore, cisplatin and alkylating drugs have recently been recommended, simply because of their activity in other histologic types of ovarian cancer and non-ovarian gynecological SCCs [7,15]. In the present study, all patients received adjuvant chemotherapy with cisplatin and mitotic disrupters such as paclitaxel and vincristine, and they showed an acceptable clinical prognosis with one recurrent case. However, like most other reports on rare diseases, our study was limited to a small number of patients, which precludes significant conclusions, but it is important to collect the cases for further review and analysis.
In summary, preoperative diagnosis of ovarian SCC-MCT is challenging since it is generally asymptomatic or manifests as non-specific abdominal pain. Radiologic and intraoperative macroscopic findings are also non-specific compared to those of mature teratomas. For patients of advanced age and with relatively large masses who are suspected to have ovarian teratomas, serum SCC-Ag levels might be a useful diagnostic tool to rule in SCC-MCTs, and adequate staging surgeries should also be considered. More evidence supporting these strategies for the management of SCC-MCT by large, multicenter studies is required.
Figures and Tables
Fig. 1
Axial transvaginal ultrasound shows a heterogeneous, echogenic, cystic, and adnexal mass measuring 12 by 8 cm in the right pelvic cavity (patient 4). There were no obvious internal multiple septa or papillary projections. The mass was preoperatively misdiagnosed as mature cystic teratoma. The arrows indicate the margin of the mass.
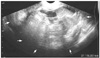
Fig. 2
Laparoscopic view showing a round, smooth, and right ovarian tumor that was filling the pelvic cavity (patient 4). Since the tumor contained sebum and hair, it was thought to be a mature cystic teratoma, and a cystectomy was performed as the primary surgery.
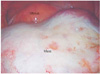
Table 1
Preoperative characteristics of the patients with ovarian SCC arising from a mature cystic teratoma
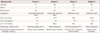
Table 2
Surgical and pathological findings

NA, not available; SCC, squamous cell carcinoma; TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; BPLND, bilateral pelvic lymph node dissection; L/S LSO, laparoscopic left salpingo-oophorectomy; PALN, paraaortic lymph node; RSO, right salpingo-oophorectomy; BPALND, bilateral paraaortic lymph node dissection; RPLND, right pelvic lymph node dissection; RPALND, right paraaortic lymph node dissection; FIGO, International Federation of Gynecology and Obstetrics.
References
1. Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997; 177:109–114.
2. Ayhan A, Bukulmez O, Genc C, Karamursel BS, Ayhan A. Mature cystic teratomas of the ovary: case series from one institution over 34 years. Eur J Obstet Gynecol Reprod Biol. 2000; 88:153–157.
3. Westhoff C, Pike M, Vessey M. Benign ovarian teratomas: a population-based case-control study. Br J Cancer. 1988; 58:93–98.
4. Comerci JT Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994; 84:22–28.
5. Singh P, Yordan EL, Wilbanks GD, Miller AW, Wee A. Malignancy associated with benign cystic teratomas (dermoid cysts) of the ovary. Singapore Med J. 1988; 29:30–34.
6. Mekaru K, Kamiyama S, Masamoto H, Yagi C, Hirakawa M, Inamine M, et al. Squamous cell carcinoma arising in an ovarian mature cystic teratoma complicating pregnancy: a case report. Arch Gynecol Obstet. 2008; 278:287–290.
7. Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, Munstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008; 9:1173–1180.
8. Kim MK, Nam EJ, Kim JW, Kim YT, Kim JH, Kim SW, et al. Squamous cell carcinoma arising from mature cystic teratoma of the ovary: a clinicopathologic analysis. Korean J Obstet Gynecol. 2006; 49:1455–1462.
9. Ji HY, Kim TJ, Kim MJ, Lee EJ, Lee YY, Kim CJ, et al. A study for diagnosis of squamous cell carcinoma arising from mature cystic teratoma. Korean J Obstet Gynecol. 2009; 52:1258–1264.
10. Kikkawa F, Nawa A, Tamakoshi K, Ishikawa H, Kuzuya K, Suganuma N, et al. Diagnosis of squamous cell carcinoma arising from mature cystic teratoma of the ovary. Cancer. 1998; 82:2249–2255.
11. Kang WD, Yun DS, Lee JY, Kim SM, Choi HS. A clinicopathologic study of 11 cases with malignant transformation arising in mature cystic teratomas. Korean J Obstet Gynecol. 2004; 47:650–655.
12. Chen P, Yeh CC, Lee FK, Teng SW, Chang WH, Wang KC, et al. Squamous cell carcinoma occurring in the pelvis after total hysterectomy and bilateral salpingo-oophorectomy for an ovarian mature teratoma with malignant transformation. Taiwan J Obstet Gynecol. 2012; 51:446–448.
13. Park SB, Cho KS, Kim JK. CT findings of mature cystic teratoma with malignant transformation: comparison with mature cystic teratoma. Clin Imaging. 2011; 35:294–300.
14. Tseng CJ, Chou HH, Huang KG, Chang TC, Liang CC, Lai CH, et al. Squamous cell carcinoma arising in mature cystic teratoma of the ovary. Gynecol Oncol. 1996; 63:364–370.
15. Omura G, Blessing JA, Ehrlich CE, Miller A, Yordan E, Creasman WT, et al. A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma: a Gynecologic Oncology Group Study. Cancer. 1986; 57:1725–1730.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download