Abstract
Objective
To investigate the clinical significance of atypical glandular cells (AGC) by analyzing the prevalence and histologic outcomes of patients with AGC according to Pap smear.
Methods
The medical records of 83 patients who were diagnosed AGC on Pap tests at the Pusan National University Hospital outpatient department and health care center from January 1998 to March 2006 were reviewed.
Results
The prevalence of AGC was 55 of 54,160 (0.10%) and 28 of 54,160 (0.05%) for AGC-not otherwise specified (NOS) and neoplastic associated AGC, respectively. The histopathologic results of the AGC-NOS group (n=55) were as follows: low-grade squamous intraepithelial lesion, 7 (12.7%); high-grade squamous intraepithelial lesion, 4 (7.2%); adenocarcinoma of cervix, 3 (5.4%); endometrial carcinoma, 2 (3.6%); and other malignancies including 2 ovarian cancer cases and 1 breast cancer case, 3 (5.4%). The histopathologic results for the AGC-associated neoplastic group (n=28) were as follows: low-grade squamous intraepithelial lesion, 1 (3.5%); high-grade squamous intraepithelial lesion, 3 (10.7%); adenocarcinoma of cervix, 5 (17.8%); endometrial carcinoma, 4 (4.8%); and additional malignancies including 3 stomach cancer cases, 2 ovarian cancer cases, and 2 breast cancer cases; 7 (25%).
Conclusion
AGCs may represent a variety of benign and malignant lesions. AGC-associated neoplastic findings may be related to gynecological or extrauterine malignancies. Thus, when AGCs, especially neoplastic AGCs, are encountered, it is best to evaluate the cervix not only for typical maladies, but also for gynecological and non-gynecological malignancies.
Among malignant tumors in Korean women, cervical cancers have been reported as the fifth most common form of cancer after breast, stomach, colorectal, and thyroid cancers [1]. Recently, however, the occurrence of cervical cancer has decreased as it takes considerable amount of time to develop invasive cancers through a progress of dysplasia and intraepithelial carcinoma, and early detection of precancerous lesions is readily available due to periodic screening and the development of cervical cancer examination methods utilizing colpos copy and human papillomavirus (HPV) tests [2].
Pap smears are the most frequently utilized method for cervical cancer screening. The Pap smear technique was first developed by Papanicolaou and Taut [3], in 1941, and although it is a convenient, inexpensive, and safe method for cervical cancer screening, the rate of false negative errors is between 6% and 55%. In order to improve upon this error rate, liquid-based Pap [4], colposcopy, and human papillomavirus tests have been suggested as alternative screening methods [5]. Likewise, 'The Bethesda System' (TBS) was established by the National Cancer Institute meeting, held in Bethesda, USA in 1988, to address problems regarding diagnostic classification of Pap smear results. Currently, a variety of classification methods are utilized around the world, especially in the US. Specifically, atypical squamous cells of undetermined significance (ASCUS) and atypical glandular cells of undetermined significance (AGUS) are among the most important characteristics of TBS. According to TBS, ASCUS is defined as foremost by abnormal cells observed are worse than either reparative or reactive cells, and secondly as cells that do not satisfy the quantitative and qualitative criteria of squamous intraepithelial lesions [6], where AGUS are defined as those cells that satisfy the range of positive reactive changes but are not enough sufficient to be diagnosed as invasive adenocarcinomas [7]. In 2001, the Bethesda III classification, the terminology of both ASCUS and AGUS, were updated as ASC and atypical glandular cell (AGC), respectively; where ASC is subdivided into ASC-US and ASC-H while AGUS is subdivided into AGC-not otherwise specified (NOS) and AGC-favor neoplastic, respectively, and are now utilized in clinical diagnoses [8].
ASCUS findings accounts for 3% to 5% of Pap smear results, although this figure has been reported to vary between 10% to 20% and 3% to 5% of the diagnosed patients possessed the risk of cervical intraepithelial neoplasia (CIN)1 and CIN2 or CIN3, respectively. A significant proportion of ASCUS patients with CIN1 are accompanied by positive HPV infection, the lesions of which have been reported to spontaneously disappear in more than 60% of cases. Thus, follow-up analysis via an outpatient clinic after confirming whether lesions of grade CIN2 or CIN3 are present in the patients who were diagnosed ASCUS appears to be an important research avenue [9-13]. Indeed, the prevalence of AGC accounts for 0.08% to 5.96% of known cases, and it has been shown that 8% of diagnosed patients are associated with malignant lesions, thereby requiring more attention and additional histological examinations and outpatient clinic follow-up for the patients clinically diagnosed with AGC [14].
Thus, the objective of this study was to investigate the clinical significance of AGC (AGC-NOS, AGC-favor neoplastic) by analyzing the final diagnosis results obtained from biopsies of female patients diagnosed with AGC based on the TBS classification method according to Pap smear.
The medical records of 83 patients who were diagnosed AGC (AGC-NOS, 55 subjects; AGC-favor neoplastic, 28 subjects) were analyzed retrospectively among 54,160 subjects who underwent Pap smear analysis in our hospital between January 1998 and March 2006 (Table 1). The average age of the patients was 49.2 ± 8.89 with a range of 33 to 74 years old. The average gravidity and parity were 3.91± 2.3 times and 2.01±1.1, respectively (Table 2).
Slides of patients with AGUS and AGC were reviewed by a pathology specialist based upon the 2001 revision of TBS III classification system (Fig. 1). Results of punch biopsy and cervical curettage in AGC patients were analyzed retrospectively.
Age, presence or absence of HPV infection, and CA-125 values during diagnosis were analyzed in patients with AGC-NOS and AGC-favor neoplastic AGC patients (Table 2).
Punch biopsy and hysterotrachelectasia were performed in patients with AGC. Depending upon indications, several examinations including conization, gastrointestinal examinations, and laparotomy were performed. The cases were classified into AGC-NOS and AGC-favor neoplastic cases, and the histologic results of the patient groups were compared (Tables 3, 4).
Among 54,160 subjects who received a Pap smear, the number of patients with AGC, AGC-NOS, and AGC-favor neoplastic cells was 83 (0.15%), 55 (0.10%), and 28 (0.05%), respectively. Of the examination performed, conventional Pap smear and liquid-based Pap (ThinPrep) were carried out in 51,510 subjects (95.1%) and 2,650 subjects (4.9%), respectively. In conventional Pap smear, AGC, AGC-NOS, and AGC-favor neoplastic were present in 64 (0.12%), 41 (0.08%), and 23 subjects (0.04%), respectively; in liquid-based Pap (ThinPrep), AGC, AGC-NOS, and AGC-favor neoplastic were observed in 19 (0.72%), 14 (0.53%), and 5 subjects (0.19%), respectively (Table 1).
The average age of the female patients was 47.3 ± 7.8 years old for AGC-NOS, 51.9 ±10.1 years old for AGC-favor neoplastic, and 49.2 ± 8.89 years old for AGC. AGC-NOS represented 3.35 ±1.9 times the average gravidity and 1.79 ±1.0 times the average parity; AGC-favor neoplastic represented 5.15 ± 2.4 times the average gravidity and 2.51 ±1.2 times the average parity; and AGC represented 3.91± 2.3 times the average gravidity and 2.01±1.1 times the average parity. For AGC-NOS cases, HPV examination was performed in 16 subjects out of 55 cases; where 5/16 (31.2%) subjects (type 33, 1 subject; type 52, 1 subject; and type 53, 2 subjects) were deemed at high risk of infection and 1/16 (6.3%) subjects (type 6, 1 subject) were deemed at low risk of infection. In the case of AGC-favor neoplastic, evidence of a high risk of infection was found in 5/10 (50.0%) subjects (type 18, 1 subject; type 33, 2 subjects; type 35, 1 subject; type 53, 1 subject). In patients with AGC, high risk and low risk of HPV infection was present in 9/26 (34.6%) subjects and 1/26 (3.8%) subjects, respectively. During diagnosis, the CA-125 (U/mL) average was 24.4 ± 36.5 (U/mL) in 31 AGC-NOS cases, 97.6 ±155.4 (U/mL) in 19 AGC-favor neoplastic cases, and 50.8 ±101.6 for AGC cases (Table 2).
Histologic results and clinical findings diagnosed during follow-up in the patients with AGC-NOS and AGC-favor neoplastic were described as follows. For AGC-NOS cases, benign pathology indicated by normal histologic findings or benign disease was observed in 34/55 (61.8%) subjects; low grade squamous intraepithelial lesion, high grade squamous intraepithelial lesion, carcinoma in situ, adenocarcinoma in situ, and endometrial hyperplasia, which corresponded to pre-malignancy, were found in 13/55 (23.6%) subjects; and malignant pathology was observed in 8/55 (14.6%) subjects. As primary sites of the malignancy, cervical adenocarcinomas, endometrial cancer, breast cancer, and ovarian cancer were observed in 3/55 (5.4%), 2/55 (3.6%), 1/55 (1.8%), and 2/55 (3.6%) subjects, respectively. For AGC-favor neoplastic cases, benign pathology, pre-malignant, and malignant pathology were observed in 7/28 (25.0%), 5/28 (17.9%), and 16/28 (57.1%) subjects, respectively. As primary sites of the malignant, cervical adenocarcinomas, endometrial cancers, breast cancers, ovarian cancers, and stomach cancers were represented in 5/28 (17.8%) subjects, 4/28 (14.2%) subjects, 2/28 (7.1%) subjects, 2/28 (7.1%) subjects, and 3/28 (10.7%) subjects, respectively (Table 3, Fig. 2).
The relative distribution of malignant diseases in AGC-NOS and AGC-favor neoplastic was as follows. When patients were diagnosed with AGC-NOS, the observed distribution of patients was 8/55(14.6%) with malignant disease, 3/8 (37.5%) with cervical adenocarcinoma, 2/8 (25%) with endometrial cancer, 2/8 (25%) with ovarian cancer, and 1/8 (12.5%) with breast cancers. When patients were diagnosed with AGC-favor neoplastic, the observed distribution of patients was 16/28 (57.1%) with malignant disease, 5/16 (31.2%) with cervical adenocarcinoma, 4/16 (25%) with endometrial cancer, 3/16 (18.7%) with stomach cancer, 2/16 (12.5%) with ovarian cancer, and 2/16 (12.5%) with breast cancer (Table 3, Fig. 3).
Of AGCs, the relative distribution of AGC-NOS and AGC-favor neoplastic in the 10 cases with malignant disease found in extrauterine tissues at the time of diagnosis was as follows: ovarian cancer (2/10 subjects, 20%) and breast cancer (1/10 subjects, 10%) were observed in AGC-NOS, while ovarian cancer (2/10 subjects, 20%) and stomach cancer (2/10 subjects, 20%) were observed in AGC-favor neoplastic. As a result of the follow-up during the study period, malignancy was not observed in AGC-NOS, whereas malignancy was found in AGC-favor neoplastic including 1/10 subjects (10%) with stomach cancer and 2/10 subjects (20%) with breast cancers (Table 4).
Pap smears are a meaningful diagnostic tool for identifying early stages of precancerous lesions of cervical cancers, thereby reducing cancer mortality. Thus, when examination results indicate the presence of cell abnormalities accurate interpretation and subsequent follow-up is critical. In the present study, we aimed to analyze the meaning of Pap smear results in females who diagnosed as AGC (NOS, favor-neoplastic) among the females who had abnormal Pap smear results during regular check-ups. A total of 83 of 54,160 subjects (0.15%) were diagnosed with AGC, which was in agreement with a previously reported value of 0.08% to 5.96% [14], indicating that AGC diagnosis our hospital can be considered relatively well managed.
A total of 64/51,510 subjects (0.12%) and 19/2,650 subjects (4.9%) were diagnosed via conventional Pap and liquid-based Pap (ThinPrep), respectively. AGC-NOS was present in 41/51,510 subjects (0.08%) for conventional Pap and 14/2,650 subjects (0.53%) for liquid-based Pap. In addition, AGC-favor neoplastic were observed in 23/51,510 subjects (0.04%) for conventional Pap and 5/2,650 subjects (0.19%) for liquid-based Pap. Given these results, diagnosis of AGC (NOS, favor-neoplastic) was relatively more frequent in liquid-based Pap than that of conventional Pap (P=0.011), and thus AGC diagnosis utilizing liquid-based Pap appeared to increase the frequency of diagnosis. However, as liquid-based Pap accounted for only 2,650/54,160 subjects (4.9%), it is somewhat unreasonable to determine an overall diagnosis ratio, and more results are needed to support whether this observation is clinically relevant.
Since it has been reported that HPV18 is highly associated with cervical adenocarcinoma [15], we investigated the correlation of HPV infection. In AGC, HPV infection was present in 10/26 subjects (38.4%) including 1 case of low risk infection and 9 cases of high risk of infection. In cases of high risk HPV infection, 5/10 subjects (50%) and 4/16 subjects (25%) exhibited AGC-favor neoplastic and AGC-NOS, respectively, indicating that the high risk infection was significantly higher in AGC-favor neoplastic compared with AGC-NOS. Of the cases of HPV observed, type 18 accounted for 1 case, while type 33 accounted for 3 cases; these represent the most common types of HPV infections. Even though these results represent different patterns within cervical adenocarcinoma, evaluation of more subjects is required to determine whether there is a direct correlation, due to the small number of patients in this study.
The average CA-125 of patients with AGC was 50.8 ±101.6 (U/mL), whereas the average CA-125 in AGC-NOS and AGC-favor neoplastic was 24.4 ± 36.5 (U/mL) and 97.6 ±155.4 (U/mL), respectively. Although the average CA-125 was higher in AGC-favor neoplastic, it was difficult to establish whether these values were significant due to the large standard deviation.
Chhieng et al. [16], reported that AGUS (AGC) is highly correlated to malignant diseases (15.45%). Moreover, Seok et al. [17], reported that invasive diseases were found in 50 subjects (57.4%) during histologic examination, among 87 subjects who were diagnosed with AGUS by Pap smear. In the present study, 24/83 subjects (28.9%) were diagnosed with malignancy in the histologic examination results of the patients with AGC, indicating high correlation. Particularly, the frequency of AGC-favor neoplastic (16/28 subjects, 57.1%) was significantly higher than that of AGC-NOS (8/55 subjects, 14.6%), indicating that AGC-favor neoplastic was relatively more correlated to malignant diseases (P=0.000). In patients with AGC, cervical adenocarcinoma (8/83 subjects, 9.6%) was the most frequently observed malignant disease, followed by, in decreasing order, endometrial cancer (6/83 subjects, 7.2%), ovarian cancer (4/83 subjects, 4.8%), breast cancer (3/83 subjects, 3.6%), and stomach cancer (3/83 subjects, 3.6%). Recently, Schnatz et al. [18,19], and Sharpless et al. [20], also found that malignancy in extrauterine organs, primarily the large intestine and breast, can be observed in histologic examination results of patients with AGUS.
Based upon the results above, liquid-based Pap smear, colposcopy and biopsy, human papillomavirus tests, and cervical curettage are needed in patients with AGC to obtain appropriate examination results; cervical curettage may be especially important for patients who aged 35 years and older, or who possess risk factors for endometrial cancer. Moreover, examination of abnormalities within the pelvis, including pelvic examination and pelvic sonography, is also necessary.
If no abnormalities are observed according the examinations described above, additional evaluation of extrauterine adenocarcinoma such as breast examination, gastroscopy, and colonoscopy is recommended, with consideration of the patient's age. Regardless of such comprehensive examinations, a 4.7% false negative error rate has been reported [18]. Thus, it is suggested that follow-up be carried out with Pap smear every 4 to 6 months, even if patients appear to be normal according to the examinations listed above.
Given the results of the study, we can speculate that AGC, especially in cases of AGC-favor neoplastic, may be associated with adenocarcinoma (e.g., ovarian cancers, breast cancers, stomach cancers) or certain modifications found before or after AGC formation. Large-scale investigations with more subjects and investigation of the molecular basis for these results are warranted.
Figures and Tables
Fig. 1
(A) Atypical endocervical cells, not otherwise specified (cervical smears by Pap stain, 34-year-old woman). Groups of cells show round to oval nuclei with nuclear enlargement, small nucleoli, and smooth nuclear membrane. Follow-up revealed chronic cervicitis (×400). (B) Atypical endocervical cells, favor neoplastic (cervical smears by Pap stain, 67-year-old woman). Sheet of cells show enlarged nuclei with hyperchromasia, some variation in nuclear size, small nucleoli, and feathering. Follow-up revealed endocervical adenocarcinoma (×400). (C) Atypical endometrial cells, favor neoplastic (cervical smears by Pap stain, 53-year-old woman). A small group of cells have mildly hyperchromatic nuclei, small nucleoli, and a vacuolated cytoplasm. Histologic diagnosis was endometrial adenocarcinoma (×400).
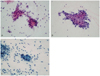
Fig. 2
Relative distribution of benign pathology, pre-malignant diseases, and malignant diseases after clinical follow-up in AGC-NOS (A) and AGC-favor neoplastic (B). AGC, atypical glandular cell; NOS, not otherwise specified.

Fig. 3
Relative distribution of malignancies after clinical follow-up in AGC-NOS (A) and AGC-favor neoplastic (B). AGC, atypical glandular cell; NOS, not otherwise specified.

References
1. Central cancer registry center in Korea. Annual report of Korean central cancer registry (2002.1-2002.12). 2003. Goyang: Korean Central Cancer Registry Center, Ministry of Health & Welfare.
2. SOG Gynecologic Oncology Committee. Annual report of gynecologic cancer registry program in Korea for 2004 (Jan. 1st, 2004-Dec. 31st, 2004). Korean J Obstet Gynecol. 2007. 50:28–78.
3. Papanicolaou GN, Taut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstet Gynecol. 1941. 42:193–206.
4. Fremont-Smith M, Marino J, Griffin B, Spencer L, Bolick D. Comparison of the SurePath liquid-based Papanicolaou smear with the conventional Papanicolaou smear in a multisite direct-to-vial study. Cancer. 2004. 102:269–279.
5. Wright TC Jr. Cervical cancer screening in the 21st century: is it time to retire the PAP smear? Clin Obstet Gynecol. 2007. 50:313–323.
6. Collins LC, Wang HH, Abu-Jawdeh GM. Qualifiers of atypical squamous cells of undetermined significance help in patient management. Mod Pathol. 1996. 9:677–681.
7. The Bethesda System for reporting cervical/vaginal cytologic diagnoses: revised after the second National Cancer Institute Workshop, April 29-30, 1991. Acta Cytol. 1993. 37:115–124.
8. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002. 287:2114–2119.
9. Wright TC, Sun XW, Koulos J. Comparison of management algorithms for the evaluation of women with low-grade cytologic abnormalities. Obstet Gynecol. 1995. 85:202–210.
10. Lonky NM, Navarre GL, Saunders S, Sadeghi M, Wolde-Tsadik G. Low-grade Papanicolaou smears and the Bethesda system: a prospective cytohistopathologic analysis. Obstet Gynecol. 1995. 85:716–720.
11. Kinney WK, Manos MM, Hurley LB, Ransley JE. Where's the high-grade cervical neoplasia? The importance of minimally abnormal Papanicolaou diagnoses. Obstet Gynecol. 1998. 91:973–976.
12. Cox JT. Management of atypical squamous cells of undetermined significance and low-grade squamous intra-epithelial lesion by human papillomavirus testing. Best Pract Res Clin Obstet Gynaecol. 2001. 15:715–741.
13. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998. 92:727–735.
14. Meath AJ, Carley ME, Wilson TO. Atypical glandular cells of undetermined significance. Review of final histologic diagnoses. J Reprod Med. 2002. 47:249–252.
15. Bulk S, Berkhof J, Bulkmans NW, Zielinski GD, Rozendaal L, van Kemenade FJ, et al. Preferential risk of HPV16 for squamous cell carcinoma and of HPV18 for adenocarcinoma of the cervix compared to women with normal cytology in The Netherlands. Br J Cancer. 2006. 94:171–175.
16. Chhieng DC, Elgert P, Cohen JM, Cangiarella JF. Clinical implications of atypical glandular cells of undetermined significance, favor endometrial origin. Cancer. 2001. 93:351–356.
17. Seok WI, Lee KB, Lee JM, Nam UG, Yoon SJ, Choi SL, et al. Clinical analysis of atypical glandular cells of undetermined significance (AGUS) on Pap smear according to menopausal status. Korean J Obstet Gynecol. 2002. 45:967–971.
18. Schnatz PF, Guile M, O'Sullivan DM, Sorosky JI. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol. 2006. 107:701–708.
19. Schnatz PF, Pattison K, Mandavilli S, Fiel-Gan M, Elsaccar OA, O'Sullivan DM, et al. Atypical glandular cells on cervical cytology and breast disease: what is the association? J Low Genit Tract Dis. 2011. 15:189–194.
20. Sharpless KE, Schnatz PF, Mandavilli S, Greene JF, Sorosky JI. Lack of adherence to practice guidelines for women with atypical glandular cells on cervical cytology. Obstet Gynecol. 2005. 105:501–506.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download