Abstract
Primary hepatoid carcinoma of the ovary (HCO) is a rare type of ovarian tumor that resembles hepatocellular carcinoma both histologically and immunohistochemically in its staining for alpha-fetoprotein (AFP). We describe a 51-year-old woman who presented to our hospital complaining of abdominal pain. Computed tomography scan revealed a large tumor in the pelvis. She underwent total hysterectomy and bilateral salpingo-oophorectomy with tumorectomy. A right ovarian mass measuring 9×8×6 cm was found. Histological diagnosis was hepatoid carcinoma of the right ovary. But, immunohistochemically, tumor cells were not immunoreactive for AFP and there was no elevation of serum AFP level. This is the first report of an ovarian carcinoma with typical histologic features of HCO with negative staining for AFP and normal level of serum AFP in the world.
Primary hepatoid carcinoma of the ovary (HCO) is a rare alpha-fetoprotein (AFP)-producing ovarian tumor which is histologically similar to a hepatocellular carcinoma (HCC). In 1987, Ishikura and Scully [1] first proposed the conception of a "hepatoid carcinoma" and described HCO which had tumor cells with abundant eosinophilic cytoplasm which satisfies morphologic criteria of HCC and positive staining for AFP by immunohistochemistry. Positive staining for AFP was considered to be an essential feature of this tumor [2]. However, Ishikura and Scully [1] suggested that some ovarian carcinomas with typical histologic features of HCO with negative staining for AFP is also belonged in the HCO category. However, such an ovarian tumor has not been described. Recently, we encountered on ovarian carcinoma appropriate for morphologic criteria of HCC but do not stain positive for AFP.
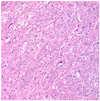
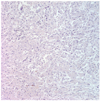
A 51-year-old woman presented to the department of internal medicine with left lower quadrant pain and hematochezia for a month. Colonoscopy revealed hyperemic mass with spontaneous bleeding at about 20 cm above the anal verge which almost obstructs the lumen. A computed tomography (CT) scan of the abdomen showed 12.5×7.8×11.2 cm sized large heterogeneous pelvic mass invading sigmoid colon and uterus (Fig. 1). There was peritoneal seeding and multiple metastatic lymphadenopathy along the bilateral iliac chain and retroperitoneal space up to level of left renal vein but the liver was normal in size and texture. Laboratory analysis revealed normal renal and liver function. The level of CA-125 was elevated to 37.5 U/mL but carcinoembryonic antigen (CEA) and CA-19-9 was normal. She was transferred to the department of Gynecology to rule out ovarian malignancy and positron emission tomography (PET) scan was done. PET scan showed large mass with heterogeneous FDG uptake in the pelvic cavity and multiple hypermetabolic seeding nodules, suggesting peritoneal seeding. The left supraclavicular lymphadenoapathy was seen and fine needle aspiration biopsy revealed metastatic carcinoma. She underwent an explorative laparotomy with total abdominal hysterectomy, bilateral salpingo-oophorectomy, appendectomy and tumorectomy including sigmoid colon with Hartmann's operation. The white-pink, granular appearance 9×8×6 cm sized cancerous mass with necrosis was found posterior to uterus which invades sigmoid colon. Frozen biopsy of the pelvic mass showed poorly differentiated carcinoma but the cell type was unknown because of severe inflammation. Bilateral pelvic lymph nodes were enlarged on palpation however, dissection was not performed. Histologically, the tumor was composed of solid sheets or aggregates of uniform cells with moderate or abundant eosinophilic cytoplasm, distinct cell borders, and centrally located nuclei with prominent nucleoli (Fig. 2). On immunohistochemical staining, p53, p16 was positive; hepatocyte paraffin-1, caudal type homeobox-2, Wilms Tumor-1, estrogen receptor and AFP was negative (Fig. 3). The tumor was relatively homongeneous, with no evidence of conventional germ cell tumor or ovarian surface epithelial tumor morphology. It was consistent with hepatoid carcinoma of the right ovary. After the surgery the CA-125 increased from 37.5 to 43.2 U/mL. But the level of serum AFP was 2.2 ng/mL (normal range, 0.0 to 10.0 ng/mL). Chemotherapy was started with an ovarian cancer regimen of paclitaxel 175 mg/m2 and carboplatin with an area under the curve of 5 every 3 weeks. Serum CA-125 returned to normal range after the first cycle of chemotherapy and remained within normal range (24 to 27 U/mL) during three cycles of chemotherapy. A CT scan after the three cycles of chemotherpy showed progression of disease with 4.3 cm recurrent peritoneal carcinomatosis in pelvic cavity and extensive metastatic lymphadenopathy with necrotic change in retroperitoneum, bilateral iliac chain and retrocrural area. Second line treatment with docetaxel was given three cycles but the disease progressed rapidly and ileus developed due to seeding mass in the abdominal cavity and pulmonary effusion newly developed. The patient expired 6 months after the initial diagnosis.
Primary HCO is a rare AFP-producing undifferentiated epithelial carcinoma that is similar to HCC histologically. HCO has been described in women between the ages of 42 to 78 years, with a mean age at diagnosis of 62 years [2]. Though HCO is usually associated with an elevation in serum AFP, these carcinomas are not associated with germ cell neoplastic components or gonadal dysgenesis [1]. Serum CA-125 is also elevated although less than the AFP.
Grossly, the tumor may appear as entirely solid or with cystic areas and there may be multiple foci of hemorrhage and necrosis [3]. Microscopically, HCO resembles hepatocellular carcinoma appearing as tumor cells arranged in sheets, and has abundant eosionophillic cytoplasms with central nuclei and distinct cellular borders [4]. On immunostaining, the tumor is focally positive for AFP and polyclonal CEA and is positive for cytokeratin [4].
The differential diagnoses of HCO include hepatoid yolk sac tumor (HYST), clear-cell carcinoma, lipid cell tumor, endometrial carcinoma and undifferentiated carcinoma [1]. HYST is another AFP producing tumor with hepatoid differentiation which is very similar to HCO. HYST occurs in a younger population between the ages of 7 to 54 years, with a mean age at diagnosis of 22 years and is regarded as a germ-cell tumor and is characterized by uniform-appearing tumor cells. HCO usually occurs in an older population between the ages of 42 to 78 years (mean age, 63 years) and is not associated with germ cell neoplastic components or gonadal dysgenesis and has histological feature of pleomorphic tumor cells with abundant eosinophilic cytoplasm. In case of this patient, HYST was unlikely since HYST occurs at a younger age (30 years), is associated with gonadal dysgenesis, and usually contains other germ cell tumors [5]. HYST is hepatocyte paraffin-1 negative and focally positive for CEA. Our case was also negative for hepatocyte paraffin-1 but gonadal dysgenesis was not seen (Fig. 4).
It is difficult to distinguish metastatic hepatocellular carcinoma from HCO. Hepatocellular carcinoma rarely metastasizes to the ovary [2]. And elevation of CA-125, a marker for ovarian surface epithelial tumors would support an ovarian origin. In the case of our patient, CA-125 was slightly elevated and AFP level was even within normal range. And there was no evidence of an intrahepatic lesion in a pre-operative abdomino-pelvic CT or during an operative inspection. Therefore, metastatic HCC was excluded.
HCO is basically an AFP-producing undifferentiated epithelial carcinoma and the diagnosis of HCO was established on the basis of classic histopathologic findings and immunohistochemical staining pattern. Usually tumor cells of HCO are positive for AFP staining and the presence of focal staining for AFP was considered to be essential when diagnosing AFP. However, not all HCO are associated with AFP positive, AFP is negative in some cases. Undifferentiated carcinoma with abundant eosinophillic cytoplasm which is negative for AFP staining is also considered as hepatoid carcinoma [2]. Two cases of HCO have been reported in Korea, and both of them were immunoreactive for AFP staining and serum AFP was elevated [6,7]. Nishida et al. [2] described a case of ovarian hepatoid carcinoma without staining for AFP in primary site. However, AFP staining was positive in metastatic lesion (uterine corpus). In case of our patient, the tumor of ovary, the primary site, was negative for AFP staining, but histologically the tumor was composed of solid sheets or aggregates of uniform cells with moderate or abundant eosinophilic cytoplasm, distinct cell borders, and centrally located nuclei with prominent nucleoli that is typical histologic feature of hepatoid carcinoma. Ishikura and Scully [1] suggested that some ovarian carcinomas with typical histologic features of a hepatoid carcinoma belonged in hepatoid carcinoma category despite their failure to stain for AFP. AFP is an oncodevelopmental protein, produced by the yolk sac, fetal liver and hepatocellular carcinoma and germ cell tumors composed of yolk sac tumor components in adults. Since the production of AFP is closely coupled to cellular division and the degree of cell differentiation, the capability of AFP production by neoplastic cells is transient. Hence, it is reasonable to suggest that positive staining for AFP is not always essential for the diagnosis of AFP producing tumors [2].
HCO is a rare but highly aggressive tumor of uncertain origin. Usually patients with HCO present with lower abdominal pain and progressive abdominal distension. But it can also be detected as an asymptomatic unilateral ovarian mass. HCO commonly progresses rapidly, with metastases in the abdomen and occasionally to the lungs and the patients died within 2 years [2]. Most patients have been treated with ovarian cancer chemotherapy regimens, such as carboplatin and paclitaxel, with shortlived responses [8]. This was also the case with our patient who despite having an initial normal range of AFP and initial decrease in CA-125 developed progression after three cycles of chemotherapy. After disease progression on first-line regimen, we changed regimen to docetaxel but this drug did not lead to a response in this patient. This suggests that the pathological findings are more important than the level of surface epithelial markers such as AFP or CA-125.
Figures and Tables
Fig. 1
A computed tomography scan of the abdomen showed 12.5×7.8×11.2 cm sized large heterogeneous pelvic mass invading sigmoid colon and uterus.
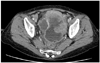
Fig. 2
The tumor was composed of solid sheets or aggregates of uniform cells with moredate or abundant eosinophilic cytoplasm, distinct cell borders, and centrally located nuclei with prominent nucleoli (H&E, ×200).
References
1. Ishikura H, Scully RE. Hepatoid carcinoma of the ovary. A newly described tumor. Cancer. 1987. 60:2775–2784.
2. Nishida T, Sugiyama T, Kataoka A, Ushijima K, Ota S, Iwanaga S, et al. Ovarian hepatoid carcinoma without staining for alpha-fetoprotein in the primary site. Int J Gynecol Cancer. 1995. 5:314–318.
3. Tochigi N, Kishimoto T, Supriatna Y, Nagai Y, Nikaido T, Ishikura H. Hepatoid carcinoma of the ovary: a report of three cases admixed with a common surface epithelial carcinoma. Int J Gynecol Pathol. 2003. 22:266–271.
4. Pandey M, Truica C. Hepatoid carcinoma of the ovary. J Clin Oncol. 2011. 29:e446–e448.
5. Kwon JE, Kim SH, Cho NH. No ancillary finding is valid to distinguish a primary ovarian hepatoid carcinoma from metastatic hepatocellular carcinoma. Int J Gynecol Cancer. 2006. 16:1691–1694.
6. Choi SM, Park CS, Oh SH, Kim TJ, Song SY, Ahn GH, et al. A case of hepatoid carcinoma of the ovary. Korean J Obstet Gynecol. 2000. 43:141–144.
7. Jang KT, Sunwoo JK, Seo SH, Kim CJ, Bae DH. A case of hepatoid carcinoma of the ovary. Korean J Obstet Gynecol. 1996. 39:427–431.
8. Trivedi P, Dave K, Shah M, Karelia N, Patel D, Wadhwa M. Hepatoid carcinoma of the ovary: a case report. Eur J Gynaecol Oncol. 1998. 19:167–169.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download