Abstract
Objective
To investigate the effect of the dimethyl sulfoxide (DMSO) and EFS-40 during vitrification on the expression of angiogenic factors in vitrified mouse ovarian tissue.
Methods
The ovarian tissues were obtained from 5 or 6 weeks aged ICR mouse. Ovarian tissues were divided into four groups: ovarian tissue without cryopreservation (control, group I), ovarian tissue vitrified with 15% DMSO (group II), ovarian tissue vitrified with EFS-40 (group III), and ovarian tissue slowly frozen with 10% DMSO (group IV). Thawing was carried out at room temperature. Levels of messenger RNA (mRNA) and protein for vascular endothelial growth factor-A (VEGF-A) and angiopoietin-2 (Angpt-2) were checked in ovarian tissues of four groups recovered on day 7 after cryopreservation. Reverse transcription-polymerase chain reaction and Western blot analysis were used to identify the levels of angiogenic factors in mouse ovarian tissues.
Results
Levels of mRNA and protein for VEGF-A and Angpt-2 were significantly decreased in cryopreserved group (group II, III and IV) than control group (group I) (P< 0.05). The significant differences of levels of mRNA and protein for VEGF-A and Angpt-2 between cryopreservation methods were observed (P< 0.05). Group III showed highest expression of mRNA and protein for VEFG-A and Angpt-2 than other cryopreservation groups (P< 0.05).
Advances in the diagnosis and treatment of childhood, adolescent and adult cancer have greatly increased the life expectancy of premenopausal women with cancer but have resulted in a growing population and adult long-term survivors of childhood malignancies [1]. The cryopreservation of ovarian tissue is a promising technique for the preservation of fecundity in young female cancer patients after sterilization by chemotherapy and/or radiotherapy [2].
Probability to be early menopause after chemotherapy, although affected by many factors, it is the most important factors were patient's age, type and cumulative dose of the drugs. Primordial follicles are most likely to be effected than growing follicles and ovarian follicle should be stopped permanently destroys while receiving chemical injury to primordial follicles [3].
The limited length of ovarian function in some ovarian transplant cases using nonvascularized grafts may partially due to ischemic injury until revascularization occurs [4]. Therefore, the establishment of an effective blood supply and the development of angiogenesis are essential for the survival of cryopreserved ovarian tissue [5]. Ovarian tissue is able to produce angiogenetic factors that promote a neovascularization after transplantation. So, revascularization plays a critical role in successful ovarian tissue transplantation.
Vascular endothelial growth factor (VEGF) was known as 1) endothelial cell mitogen factor, 2) regulate permeability of blood vessels, 3) angiogenesis in transplanted ovarian tissue [6-8]. Angiopoietins were required for regulation of angiogenesis and blood vessel stabilization [9,10]. In presence of VEGF, angiopoietin-2 (Angpt-2) enables endothelial cell migration and proliferation, promoting further angiogenesis. In the absence of VEGF, Angpt-2 blocks the recruitment of periendothelial support cells, resulting in blood vessel destabilization and regression [11-13]. Also levels of Angpt-1 and Angpt-2 may be associated with follicular growth and angiogenesis during the preovulatory period [13].
Several cryopreservation of ovarian tissue cryopreservation methods being used, but vitrification is relatively simple and does not require expensive equipment and recent research has been relatively good results are presented [14]. Several vitrification solutions were used, but the impact on the ovarian tissue was not well known. In particular, there were few studies on the effects for angiogenic factors after vitrification. Thus, the objective of this study was to determine the effect of the dimethyl sulfoxide (DMSO) and EFS-40 during vitrification on the VEGF and Angpt-2 in vitrified mouse ovarian tissue.
Female ICR mice were purchased from Koatech Co. (Pyeongtaek, Korea). The mice were housed under light-controlled and temperature-controlled conditions (12 hour of light and 12 hour of darkness; 22±2℃), and were provided with sterile food and water. Mice were treated in accordance with the standard guidelines for laboratory animal care at the animal facility of the Gyeongsang National University (GLA-090107-M0001).
Twenty ICR mice aged 5 or 6 weeks old were sacrificed, and their ovaries were placed in a large sterile Petri dish with Ham's F-10 media. The ovaries were randomly assigned into four groups (each 10 ea): 1) ovarian tissue without cryopreservation (control, group I), 2) ovarian tissue vitrified with 15% DMSO (group II), 3) ovarian tissue vitrified with EFS-40 (group III), and ovarian tissue slowly frozen with 10% DMSO (group IV).
Ovarian tissues to be vitrified were immersed in an equilibration solution (EG-20) for 2, 5, and 10 minutes that consisted of 20% ethylene glycol (EG) in Dulbecco's phosphate-buffered saline (m-DPBS) with 10% fetal bovine serum (FBS), followed by immersion in a vitrification solution (15% DMSO in group II or EFS-40 in group III) for 2 minutes. EFS-40 was consisted of 40% EG (v/v), 18% ficoll (w/v), 0.5 mol/L sucrose, and 20% FBS in m-DPBS in group III. Then, the ovarian tissue were placed onto an electron microscope gird and submerged immediately in liquid nitrogen. After 1 week, the three step cryoprotectant dilution method (0.75 M, 0.5 M, 0.25 M sucrose) was performed to thaw the ovarian tissue.
Ovarian tissues were transferred to a cryovial containing the cryoprotective mixture (Leibovitz L-15 medium, 10% FBS, and 10% DMSO) for controlled freezing using Planar Cryochamber. Cooling was started at 4℃ and continue at 2℃/min until ice nucleation was induced at -7℃. The temperature was then reduced at 2℃/min until -35℃ and subsequently at 25℃/min until -140℃ before the cryovials were plunged into liquid nitrogen. After 1 week, thawing was carried out at room temperature.
Total RNA was isolated from ovarian tissues with TRIZOL (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. One microgram of total RNA was reverse-transcribed using SuperScript III RT (Invitrogen), random hexamers (50 pmol), and dNTPs (2.5 mM) at 37℃ for 20 minutes. Specific sequence of primer for VEGF-A, Angpt-2 was obtained from Gene Bank Database (Table 1). PCR reactions were carried out with the following programs, first heated to 94℃ for 4 minutes, then 30 cycles of denaturation at 94℃ for 40 second, annealing at 55℃ for 40 second, and extension at 72℃ for 40 second, and then final elongation step at 72℃ for 8 minutes. PCR products were analyzed by 2% agarose gel electrophoresis. Expression of each messenger RNA (mRNA) species was normalized to that of glyceraldehyde-3-phosphate dehydrogenase. Relative quantitation of the target gene expression was evaluated using SigmaGel software (Sigma, St. Louis, MO, USA).
Mouse ovarian tissues were preserved in Pro-prep (iNtRON, Seongnam, Korea) solution until analysis. Proteins were separated by SDS-PAGE in 8% to 12% gels and blotted onto polyvinylidene difluoride membrane. The blots were blocked with 5% skim-milk in TBST (20 mM Tris-buffered saline and 0.05% Tween 20, pH 7.5) at room temperature for 1 hour and incubated overnight at 4℃ with VEGF-A (1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), angpt-2 (1:1,000; Santa Cruz Biotechnology Inc.) and β-actin (1:10,000; Sigma) anti-VEGF antibody (1:500). Blots were then washed and incubated with secondary antibody, followed by washing and detection of immunoreactivity with enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA). For quantitation of the result, each band density was read by SigmaGel software (Sigma). Each protein expression level was the normalized relative quantitative values using each corresponding β-actin expression level as an internal control.
The data were represented as the mean ± standard error. Statistical analyses were performed using PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA). Non-parametric Kruskal-Wallis test was used and Tukey test using ranks for post-hoc analysis. The level of significance was set at P<0.05.
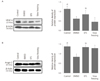
The expressions of VEGF-A and Angpt-2 were significantly increased in group-III than in other cryopreservation groups (group II and IV) (P<0.05) (Fig. 2). In vitrification group, EFS-40 used as vitrification solution (group III) showed significantly increased levels of VEGF-A and Angpt-2 than 15% DMSO (group II) (P<0.05) (Fig. 2).
Ovarian tissue has been successfully cryopreservated and transplanted into rodents, rabbits, sheep, and monkeys [15-17]. The problem to be solved was fall in the number of primodial follicles in ovarian tissue is due to hypoxia and vascular injury, probably resulting from osmotic damage or chemical toxicity from cryoprotectant agents [18]. The loss of primodial follicles in cryopreserved ovarian tissue after transplantation is estimated to be 50% to 65% [19,20] in some studies and >90% in one study [21].
VEGF was defined characterized for its ability to induce vascular leak and permeability, as well as for its ability to promote vascular cell proliferation [22]. Incubation with hyalunonan and VEGF of human ovarian tissue can improves graft survival [23]. So, VEGF is an important factor that can predict the success of the transplantation of cryopreserved ovarian tissue. In this experiment, the reduction of VEGF-A occurred after the all of cryopreservation method. We suggested that lower reduction of VEGF-A in vitrification using EFS-40 than vitrification using DMSO and slow-freezing should be to contribute to the success of transplantation of the cryopreserved ovarian tissue.
Slow freezing of ovarian tissue has been indicated because of the preservation of cell morphology and hormone production after freezing-thawing of ovarian tissue [24]. However, vitrification of ovarian tissue also resulted in preserved follicular morphology and viability and stromal density [25,26]. Indeed, several studies have shown no differences when slow freezing and vitrification were compared regarding tissue revascularization after transplantation [27]. In present study, the differences of angiogenic factors expression were observed between the vitrification using EFS and slow freezing groups. Also, the reduction of angiogenic factors has been observed when using DMSO when compared to EFS-40 as vitrification cryoprotectant. Such a decrease might be caused by the osmotic stress to which cells are exposed during vitrification and toxicity of cryoprotectants. So, vitrification could be offered as an alternative method to reduce the damage of angiogenic factors while cryopreservation of ovarian tissue and EFS-40 could be efficient vitrification solution for preservation of VEGF-A and Angpt-2 during vitrification of mouse ovarian tissue. Future studies should investigate vitrification solution for improving preservation of angiogenic factors that should be essential role for revascularization after implantation of cryopreserved ovarian tissue.
Figures and Tables
Fig. 1
Reverse transcription-polymerase chain reaction analysis of mRNA levels for (A) VEGF-A and (B) Angpt-2 after variable cryopreservation methods and quantitative analysis of each group. GAPDH was used as internal standard. The data represents mean±standard error of three independent experiments. DMSO, dimethyl sulfoxide; EFS, 40% EG, 18% ficoll, 0.5 mol/L sucrose, and 20% FBS in modified Dulbecco's phosphate buffered saline; Angpt-2, angiopoietin-2; VEGF-A, vascular endothelial growth factor-A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. a)P<0.05 vs. cryopreservation groups (vitrification using DMSO, EFS, and slow freezing; b)P<0.05 vs. other cryopreservation groups (vitrification using DMSO and slow freezing).

Fig. 2
Western blot analysis of (A) VEGF-A and (B) Angpt-2 after variable cryopreservation methods and quantitative analysis of each group. β-Actin was used as internal standard. The data represents mean±standard error of three independent experiments. DMSO, dimethyl sulfoxide; EFS, 40% EG, 18% ficoll, 0.5 mol/L sucrose, and 20% FBS in modified Dulbecco's phosphate buffered saline; Angpt-2, angiopoietin-2; VEGF-A, vascular endothelial growth factor-A. a)P<0.05 vs. cryopreservation groups (vitrification using DMSO, EFS, and slow freezing); b)P<0.05 vs. other cryopreservation groups (vitrification using DMSO and slow freezing).
Acknowledgments
This work (RPP-2009-048) was supported by the fund of Research Promotion Program, Gyeongsang National University, 2009.
References
1. Blatt J. Pregnancy outcome in long-term survivors of childhood cancer. Med Pediatr Oncol. 1999; 33:29–33.
2. Aubard Y, Poirot C, Piver P, Galinat S, Teissier MP. Are there indications for ovarian tissue cryopreservation? Fertil Steril. 2001; 76:414–415.
3. Lo Presti A, Ruvolo G, Gancitano RA, Cittadini E. Ovarian function following radiation and chemotherapy for cancer. Eur J Obstet Gynecol Reprod Biol. 2004; 113:Suppl 1. S33–S40.
4. Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001; 286:1490–1493.
5. Bedaiwy MA, Jeremias E, Gurunluoglu R, Hussein MR, Siemianow M, Biscotti C, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003; 79:594–602.
6. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000; 407:242–248.
7. Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999; 5:1115–1121.
8. Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999; 13:1055–1066.
9. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996; 87:1171–1180.
10. Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000; 6:460–463.
11. Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999; 18:5356–5362.
12. Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998; 83:233–240.
13. Nishigaki A, Okada H, Tsuzuki T, Cho H, Yasuda K, Kanzaki H. Angiopoietin 1 and angiopoietin 2 in follicular fluid of women undergoing a long protocol. Fertil Steril. 2011; 96:1378–1383.
14. Milenkovic M, Diaz-Garcia C, Wallin A, Brannstrom M. Viability and function of the cryopreserved whole rat ovary: comparison between slow-freezing and vitrification. Fertil Steril. 2012; 97:1176–1182.
15. Candy CJ, Wood MJ, Whittingham DG. Follicular development in cryopreserved marmoset ovarian tissue after transplantation. Hum Reprod. 1995; 10:2334–2338.
16. Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J. Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertil Steril. 2002; 77:403–408.
17. Almodin CG, Minguetti-Camara VC, Meister H, Ferreira JO, Franco RL, Cavalcante AA, et al. Recovery of fertility after grafting of cryopreserved germinative tissue in female rabbits following radiotherapy. Hum Reprod. 2004; 19:1287–1293.
18. Yin H, Wang X, Kim SS, Chen H, Tan SL, Gosden RG. Transplantation of intact rat gonads using vascular anastomosis: effects of cryopreservation, ischaemia and genotype. Hum Reprod. 2003; 18:1165–1172.
19. Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196 C. Endocrinology. 1999; 140:462–471.
20. Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J. Histologic and ultrastructural evaluation of fresh and frozen-thawed human ovarian xenografts in nude mice. Fertil Steril. 2000; 74:122–129.
21. Aubard Y, Piver P, Cogni Y, Fermeaux V, Poulin N, Driancourt MA. Orthotopic and heterotopic autografts of frozen-thawed ovarian cortex in sheep. Hum Reprod. 1999; 14:2149–2154.
22. Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999; 237:1–30.
23. Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, et al. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012; 27:474–482.
24. Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, et al. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007; 55:261–268.
25. Santos RR, Tharasanit T, Van Haeften T, Figueiredo JR, Silva JR, Van den Hurk R. Vitrification of goat preantral follicles enclosed in ovarian tissue by using conventional and solid-surface vitrification methods. Cell Tissue Res. 2007; 327:167–176.
26. Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009; 24:1670–1683.
27. Rahimi G, Isachenko V, Kreienberg R, Sauer H, Todorov P, Tawadros S, et al. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Eur J Obstet Gynecol Reprod Biol. 2010; 149:63–67.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download