Abstract
Objective
Although there is a large body of data on acute hepatitis A virus (HAV) worldwide, data regarding the occurrence of HAV during pregnancy is limited. It is commonly acknowledged that HAV is not associated with severe outcomes or complications during pregnancy. In contrast, there are several reported cases of vertical HAV transmission. Moreover, it has been recently reported that HAV infection during pregnancy is associated with gestational complications. In Korea, the incidence of HAV infection has increased from 317 cases in 2002 to 13,117 cases in 2009. However, HAV infection during pregnancy is rarely reported in Korea.
Methods
This study was conducted as a retrospective cohort series of pregnant women presenting to Korea University Medical Center between January 2000 and October 2009 in whom a diagnosis of HAV infection was made.
Hepatitis A virus (HAV) is an enterovirus of the family Picornaviridae that is transmitted primarily via the fecal-oral route. Most infections in children younger than six years of age are mild or asymptomatic. However, infections in older children and adults are usually symptomatic, with a small number (less than 1%) of patients experiencing a fulminant course of illness that may result in death or emergent liver transplantation in those with advanced or comorbid conditions [1].
HAV incidence rates have decreased dramatically in the United States due to improved hygiene, sanitation, socioeconomic conditions, and vaccination programs [2]. Poor sanitation and contaminated water supplies may lead to infection in early childhood, resulting in mild illness. Therefore, as sanitation and living standards improve and fewer young children are infected, more adults are seronegative for HAV and are therefore susceptible to infection [3,4].
Despite the large body of data on HAV worldwide, little information is available regarding HAV infection during pregnancy. HAV infection is not associated with severe outcomes or complications during pregnancy [5] and maternal-infant HAV transmission is thought to be uncommon [6]. On the other hand, Elinav et al. [3] reported that HAV infection during pregnancy is associated with gestational complications, including preterm contraction. There are also numerous reported cases of vertical HAV transmission [7-14], with two cases reporting that HAV infection was associated with meconium peritonitis and perforation of the distal ileum in the infant, requiring postnatal surgery [7,8].
Currently, Korea has a low endemicity for HAV. However, recent reports from the Korea Centers for Disease Control and Prevention show that there has been a sharp increase in incidence of HAV infection from 317 cases in 2002 to 13,111 cases in 2009 (http://www.cdc.go.kr/kcdchome/jsp/home/main/Main.jsp) (Fig. 1). This may be partially explained by a dramatic drop in seroprevalence rates among children and adolescents under 20 years of age (from over 63.8% in 1979 to 4.6% in 1996) [15]. A potential concern arising from this incidence pattern in Korea is that approximately 80% of women with HAV infection are between the ages of 20 and 39 (Fig. 2), indicating that the incidence of HAV infection during pregnancy in Korea will increase. However, to our knowledge, there are no studies investigating the relationship between HAV infection and pregnancy in Korea.
The aim of this study is to review cases of HAV infection during pregnancy and to evaluate any associated clinical characteristics and maternal and fetal complications.
This study was conducted as a retrospective cohort series of pregnant women presenting to Korea University Medical Center between January 2000 and October 2009 in whom a diagnosis of HAV infection was made. Cases were identified by reviewing the computerized diagnoses of all pregnant women who were patients at the hospital during that period of time. Comprehensive clinical and laboratory information were extracted from medical and obstetric records of all identified cases.
HAV infection was defined as positive immunoglobulin M and negative immunoglobulin G anti-HAV antibodies with acute elevation of hepatic aminotransferase levels in the absence of other etiologies for acute liver injury (including preeclampsia, hemolysis, elevated liver enzymes, low platelets [HELLP] syndrome, and acute fatty liver of pregnancy or intercurrent liver disease).
Sixteen thousand nine hundred forty four deliveries were carried out between January 2000 and October 2009 at Korea University Medical Center. Among these 12 cases of maternal HAV infection were identified. The obstetric characteristics of study sample are noted in Table 1. The mean age of the 12 HAV cases was 29.0 years (range, 25-36 years), range of parity was 0-1, and mean gestational age at diagnosis was 18.3 (range, 5.5-35.1 weeks). Five cases (41.7%) occurred in 2009.
Among the 12 patients, there were two cases of cholestatic hepatitis (cases 7 and 8), two cases of involving preterm contraction and subsequent preterm labor (cases 8 and 11), and one case of the fetus presenting with ascites and intra-abdominal calcification (case 9). In one case, the pregnant patient underwent elective termination after the diagnosis of HAV infection in early pregnancy (case 3). Vertical transmission was confirmed in case 8.
Table 2 shows the clinical and laboratory characteristics of the study sample. All cases developed prodromal symptoms, including malaise, anorexia, and nausea. Laboratory findings were consistent with hepatitis.
A 31-year-old gravida 1 para 0 presented to our hospital with fever, fatigue, malaise, pruritus, and jaundice at 15.2 weeks gestation and was diagnosed with HAV infection. Because of protracted jaundice, pruritus, and an increase in serum bilirubin level, the patient was treated with prednisone 20 mg/day. The patient subsequently experienced symptomatic relief and a rapid drop in serum bilirubin level. Prednisone was tapered. No clinical or laboratory deterioration was observed after discontinuation of therapy. She was followed up, her pregnancy continued unremarkably, and she vaginally delivered a male baby at 38.4 weeks gestation.
A 29-year-old gravida 1 para 0 was admitted at 33.2 weeks gestation with preterm contractions and premature rupture of membranes. Laboratory tests revealed abnormal liver function tests, and a diagnosis of HAV infection was eventually made. At 33.4 weeks gestation, she vaginally delivered a 2208 g male infant with Apgar scores of 8 and 10 at 1 and 5 minutes, respectively. The neonate was negative for HAV.
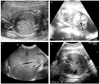
A 29-year-old gravida 1 para 0 was referred to our hospital at 19.5 weeks gestation for suspected maternal HAV infection. After the diagnosis of maternal HAV infection, sonographic examination of the fetus was performed, which revealed ascites at 20.2 weeks of gestation (Fig. 2A), and polyhydramnios and intra-abdominal calcified lesions at 22.1 weeks of gestation (Fig. 2B), indicating meconium peritonitis. However, serial sonography showed spontaneous resolution of the intra-abdominal calcified lesions, ascites, and polyhydramnios (Fig. 2C). At 40.2 weeks of gestation, the patient delivered a 3,420 g male baby vaginally with Apgar scores of 9 and 10 at 1 and 5 minutes, respectively. Three days after birth, sonography of the patient's abdomen showed no abnormalities (Fig. 2D). The baby fed uneventfully and no problems have been reported at 6 months of age.
In our sample, there were two cases of preterm contraction with subsequent preterm delivery, as in previously-reported cases [3,16]. In some previous cases of preterm labor, the exact cause was never identified [10,11]. While the exact mechanism by which HAV causes preterm contraction is not clear, there are several possible explanations. First, viral hepatitis presents with a markedly proinflammatory milieu [17]. In particular, serum levels of interleukin (IL)-1, IL-6, and tumor necrosis factor-alpha are significantly increased in patients with acute HAV infection [18]. These cytokines are also known to be associated with preterm contraction [19-22]. Therefore, preterm contraction may be precipitated by cytokine release as a result of systemic inflammatory response due to HAV infection. This is similar to the pathophysiolgic mechanisms by which pyelonephritis causes preterm contraction [3].
It has also been reported that fulminant hepatitis is associated with an increased risk of preterm labor [23]. Acute HAV infection results in metabolic abnormalities throughout the body, causing insufficient energy generation and blood and oxygen supply through the placenta. Accordingly, preterm labor is likely to develop [23]. This may be another possible explanation for the association between HAV infection and preterm contractions. Therefore, preterm contraction and preterm labor may develop due to both the direct release of inflammatory cytokines and changes in utero-placental circulation that occur as a consequence of HAV infection.
In this study, we presented the case of a fetus with ascites and intra-abdominal calcifications at around 20 weeks gestation. Interestingly, we discovered two case reports with a nearly identical time course and presentation [7,8], but these cases necessitated post-natal surgery. These infections occurred at 13 and 20 weeks gestation. Other cases of vertical transmission following HAV onset in the mother at later than 33 weeks of gestations have reported favorable neonatal outcomes [9-14] with the exception of mild neonatal jaundice [9,11,12,14]. The inconsistencies in the reported effects of maternal HAV infection on fetus may be due to varying susceptibility of the fetus to maternal infection according to gestational age. Infection early in gestation (as fetal organs are developing) can cause more severe damage or stillbirth. It has been known that the risk of congenital varicella syndrome is highest between 13 and 20 weeks gestation, with an overall incidence of 0.55% in the first trimester, 1.4% in the second trimester, and 0% in the third trimester [24]. These results suggest that the fetus may have critical periods of high susceptibility for HAV infection and that the spectrum of clinical disease that occurs may be correlated with the gestational age of the fetus.
In this study, all of the patients presented with prodromal symptoms that included malaise, anorexia, and nausea. All of these are similar to pregnancy-related symptoms, which makes it difficult to correctly diagnose and treat HAV in the early stages prior to the onset of jaundice. Considering that fertile women have lower seroprevalence rates in Korea [15] and that the HAV vaccine is safe for use in pregnancy [25], addition of HAV serology to maternal screening at the first prenatal visit and vaccination of seronegative pregnant women may eliminate the risk of HAV infection during pregnancy [3].
As only 12 cases of symptomatic women were identified during the study period, we did not perform statistical analysis for maternal or fetal complications in this study. HAV may be low relatively low in incidence compared to other infections; however, asymptomatic pregnant women and pregnant women with mild disease (with symptoms similar to those expected in normal pregnancy) may not be diagnosed. Therefore, the true incidence may be underestimated. Further studies are needed to evaluate the epidemiology of HAV infection during pregnancy and its clinical consequence in maternal and fetal aspects.
In conclusion, we present 12 cases of HAV infection during pregnancy, including cases with maternal and fetal complications. Because the incidence of HAV infection in women of reproductive age is increasing, a further focus on preventing HAV infection during pregnancy is warranted.
Figures and Tables
Fig. 1
(A) Incidence of hepatitis A virus (HAV) infection from January 2002 to October 2009 in Korea and (B) Korean women by age.

Fig. 2
Prenatal (A-C) and neonatal (D) sonography were performed in case 9. (A) Ascites was found at 20 weeks gestation (white arrow), and (B) an intra-abdominal calcification was seen at 22.1 weeks gestation (black arrow). (C) However, prenatal sonography at 38.1 weeks gestation showed (D) spontaneous resolution of the ascites and intra-abdominal calcification, which was confirmed by postnatal sonography at 3 days of life.
References
1. Kemmer NM, Miskovsky EP. Hepatitis A. Infect Dis Clin North Am. 2000; 14:605–615.
2. FitzSimons D, Hendrickx G, Vorsters A, Van Damme P. Hepatitis A and E: update on prevention and epidemiology. Vaccine. 2010; 28:583–588.
3. Elinav E, Ben-Dov IZ, Shapira Y, Daudi N, Adler R, Shouval D, et al. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology. 2006; 130:1129–1134.
4. Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004; 132:1005–1022.
5. Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev. 2001; 14:38–58.
6. Tong MJ, Thursby M, Rakela J, McPeak C, Edwards VM, Mosley JW. Studies on the maternal-infant transmission of the viruses which cause acute hepatitis. Gastroenterology. 1981; 80:999–1004.
7. Leikin E, Lysikiewicz A, Garry D, Tejani N. Intrauterine transmission of hepatitis A virus. Obstet Gynecol. 1996; 88:690–691.
8. McDuffie RS Jr, Bader T. Fetal meconium peritonitis after maternal hepatitis A. Am J Obstet Gynecol. 1999; 180:1031–1032.
9. Watson JC, Fleming DW, Borella AJ, Olcott ES, Conrad RE, Baron RC. Vertical transmission of hepatitis A resulting in an outbreak in a neonatal intensive care unit. J Infect Dis. 1993; 167:567–571.
10. Tanaka I, Shima M, Kubota Y, Takahashi Y, Kawamata O, Yoshioka A. Vertical transmission of hepatitis A virus. Lancet. 1995; 345:397.
11. Erkan T, Kutlu T, Cullu F, Tumay GT. A case of vertical transmission of hepatitis A virus infection. Acta Paediatr. 1998; 87:1008–1009.
12. Urganci N, Arapoglu M, Akyildiz B, Nuhoglu A. Neonatal cholestasis resulting from vertical transmission of hepatitis A infection. Pediatr Infect Dis J. 2003; 22:381–382.
13. Motte A, Blanc J, Minodier P, Colson P. Acute hepatitis A in a pregnant woman at delivery. Int J Infect Dis. 2009; 13:e49–e51.
14. Renge RL, Dani VS, Chitambar SD, Arankalle VA. Vertical transmission of hepatitis A. Indian J Pediatr. 2002; 69:535–536.
15. Sohn YM, Rho HO, Park MS, Park JH, Choi BY, Ki M, et al. The changing epidemiology of hepatitis A in children and the consideration of active immunization in Korea. Yonsei Med J. 2000; 41:34–39.
16. Willner IR, Uhl MD, Howard SC, Williams EQ, Riely CA, Waters B. Serious hepatitis A: an analysis of patients hospitalized during an urban epidemic in the United States. Ann Intern Med. 1998; 128:111–114.
17. Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988; 2:72–74.
18. Torre D, Zeroli C, Giola M, Ferrario G, Fiori GP, Bonetta G, et al. Serum levels of interleukin-1 alpha, interleukin-1 beta, interleukin-6, and tumor necrosis factor in patients with acute viral hepatitis. Clin Infect Dis. 1994; 18:194–198.
19. Murtha AP, Greig PC, Jimmerson CE, Herbert WN. Maternal serum interleukin-6 concentration as a marker for impending preterm delivery. Obstet Gynecol. 1998; 91:161–164.
20. Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992; 166:1576–1587.
21. Fortunato SJ, Menon RP, Swan KF, Menon R. Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol. 1996; 174:1855–1861.
22. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006; 195:1578–1589.
23. Li XM, Ma L, Yang YB, Shi ZJ, Zhou SS. Clinical characteristics of fulminant hepatitis in pregnancy. World J Gastroenterol. 2005; 11:4600–4603.
24. Tan MP, Koren G. Chickenpox in pregnancy: revisited. Reprod Toxicol. 2006; 21:410–420.
25. Bell BP, Shapiro CN, Alter MJ, Moyer LA, Judson FN, Mottram K, et al. The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies. J Infect Dis. 1998; 178:1579–1584.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download