Abstract
Objective
This study was conducted to examine the influences of supplementation of the serum substituents and available period of serum-free Vero cell conditioned media (SF-VCM) manufactured from Dulbecco's modified Eagle medium cultured with Vero cells for in vitro development of mouse preimplantation embryos.
Methods
A total of 1,099 two-cell embryos collected from imprinting control region mice were cultured in SF-VCM with 10% and 20% human follicular fluid (hFF), serum substitute supplement (SSS), and serum protein substitute (SPS). Development of embryos was observed every 24 hours. Results between different groups were analyzed by chi-square test, and considered statistically significant when P-value was less than 0.05.
Results
The rates of embryonic development cultured in SF-VCM supplemented with serum substituents were significantly higher compare with serum-free group (P < 0.05). The rates of embryonic development after 48 hours (morula≤) and 96 hours (blastocyst≤) were significantly higher in 20% SSS and 10% SPS than in 20% hFF supplementation (P < 0.05). And the rates of embryonic development after 96 hours (hatching blastocyst≤) were significantly higher in 10% SPS (94.5%) than in 20% SSS (82.6%) and 20% hFF supplementation (68.5%). The rates of embryonic development according to storage period of the SF-VCM supplemented with 10% SPS showed no significant difference between control, 2 weeks and 4 weeks group. However developmental rate in 6 weeks storage group was significantly lower than other groups.
Culture media is considered to be the most important factor for in vitro embryo culture. In particular, the composition of the culture media plays a crucial role in the development of embryos. Of the various constituents of culture media, energy sources such as glucose, pyruvate, and glutamine are the most significant factors. In 1985, Betterbed and Wright [1] reported that glucose supplementation was beneficial for ovine embryo development. Gardner and Lane [2] reported that the elimination of glucose from culture media was lethal to the development of mouse 8-cell embryos when carrying out in vitro culture until the blastocyst embryo stage. Despite such results, the positive effects of glucose tended to be ignored due to other study results, which suggested that glucose in culture media suppressed the development of embryos in animals [2-7] and human [8].
Pyruvate facilitates the development of mouse morula as an energy source. Gardner et al. [9] reported that pyruvate and glucose were essential components for in vitro human embryo development, with glucose having greater importance than pyruvate. In our previous study, culture media with low concentrations of glucose and pyruvate was more effective in mouse embryo development compared to a medium with only glutamine as an energy source [10].
Many researchers have attempted to generate similar in vitro conditions to those experienced by eggs in vivo ; however, recreating such conditions in culture media has proved difficult. In order to deal with such difficulties, coculture systems with multiple kinds of somatic cells were developed [11-13]. Cole and Paul [14] carried out the first study with regard to controlling the in vitro culture of mouse embryos to produce conditions similar to the in vivo environment. Since then, studies of coculture-utilizing feeder cell lines have been performed when implementing human in vitro fertilization and embryo transfer [11,15-17].
Although recent studies strongly suggest that coculture facilitates in vitro embryo development commercially available culture media is commonly utilized in the laboratory instead of coculture because of the inconvenience of handling cells and the time-consuming nature of coculture. Moreover, follicular fluid (FF), cord serum, or maternal serum is a required addition to most culture media in in vitro coculture systems; however, there are still discrepancies about the effects of such additives on in vitro embryo culture; and their stability has not been clearly demonstrated.
Recently, Kim et al. [18,19] developed a serum-free conditioned culture media generated from Dulbecco's modified Eagle medium (DMEM) with Vero cell culture, which overcomes the time and technical difficulties while maximizing the effects of coculture. They reported that the culture media manufactured by DMEM-G¼GP was the most effective. There is no studies which have investigated the most effective type and concentration of serum substituents to add on serum free Vero cell conditioned media (SF-VCM). In addition to the substituent type and concentration, the storage duration needs to be identified for maintaining best culture condition.
Therefore, the objectives of this study were to investigate the most effective serum substituent in embryo development, the most potent concentration of this substituent, and the influences of storage duration of culture media on in vitro culture of mouse embryos using SF-VCM.
Imprinting control region mice grown in Korea, including 4- to 5-week-old females and 15- to 20-week-old males, were used in this study. The mice were kept in a room with a 12 hours-dark cycle at 24℃. Animal diet and drinking water were provided ad libitum throughout the experiment.
Culture media containing Ham's F-10 (11550-043, Gibco, Carlsbad, CA, USA) with 10% serum substitute supplement (SSS) were used for perfusion, recovery, and washing of oviducts to recover 2-cell mouse embryos. DMEM (11885-084, Gibco) with 10% fetal bovine serum (FBS) was applied in the production and preparation of Vero cell layers.
The culture media utilized for the in vitro culture of the recovered 2-cell embryos was manufactured by mixing two different kinds of DMEM:DMEM (11966-025, Gibco; DMEM-G) containing glutamine (4 mM/L) without glucose and pyruvate, which is currently used in assisted reproductive technology, and DMEM (11885-084, Gibco; DMEM-GGP) containing glutamine (4 mM/L), glucose (5.5 mM/L), and pyruvate (1 mM/L). The DMEM-G¼P (3 volume DMEM-G+1 volume DMEM-GGP) culture media, which was found to be the most effective media in mouse embryo development when manufacturing serum-free conditioned culture media with DMEM and Vero cells in a previous study [19], was employed as the basic culture medium, and the composition is shown in Table 1. Streptomycin sulfate (Sigma, St. Louis, MO, USA), 0.025 g/L, and penicillin-G (Sigma), 0.05 g/L, were added to Ham's F-10, DMEM-G, and DMEM-GGP culture media. After the osmometer (Advanced Instruments, Norwood, MA, USA) was calibrated to 280 mOsm/kg, the media were filtered through 0.2 µm filters (Corning, Tewksbury, MA, USA) equipped with a vacuum pump and then stored at 4℃ until further analysis. All culture media utilized in this study were equilibrated in an incubator at 37℃ (Forma, Mariette, OH, USA) with 5% CO2 (Forma) more than 6 hours prior to use.
FF without residual blood was collected from the matured eggs of patients who underwent in vitro fertilization. After incubating the fluid at room temperature for 1 to 2 hours, the supernatant was collected, followed by two rounds of centrifugation at 3,500 rpm (30 minutes and 10 minutes). The supernatant was collected again and subjected to inactivation in a water bath at 56℃ for 35 minutes. The inactivated fluid was filtered through a 0.2 µm filtration device, aliquoted into 5 mL test tubes, and stored at -20℃ until further analysis.
Frozen Vero cells (2-3×106 cells/mL) were thawed and washed with DMEM (11885-084, Gibco) containing 10% FBS (26140-079, Gibco). After centrifugation, the mixture was diluted with fresh culture media and cultured in a 50 mL culture flask (3013, Falcon, Franklin Lakes, NJ, USA) for 4 to 5 days. Fresh DMEM culture media with 10% FBS was provided every 2 days until a monolayer of Vero cells was formed on the bottom of the flask. When the cell monolayer was formed, the cells were suspended by TrypLE (12604-013, Gibco) and then scraped off the bottom of the flask using a cell scraper (3010, Costar, Tewksbury, MA, USA), followed by centrifugation at 900 rpm for 30 minutes. The precipitates were diluted with cell culture freezing media (11101, Gibco), and the controlled number of cells, 2-3×106, was frozen. The frozen cells were stored in liquid nitrogen and thawed when needed.
The frozen Vero cells stored in liquid nitrogen were thawed and then cultured in a 50 mL flask, similar to the method of subculture. DMEM with 10% FBS was used in the case. When the Vero cells had formed a complete monolayer on the bottom of the flask, the culture media in the flask was removed. The Vero cells were next washed with serum-free DMEM that had been incubated for more than 6 hours in advance followed by cell suspension in 2 mL of TrypLE (12604-013, Gibco). When all Vero cells were in single cell form according to microscopic examination, all cells were separated from the bottom of the flask. The same amount of FBS was then added to the flask containing the separated Vero cells to neutralize trypsin. The separated cells were diluted by DMEM culture media with 10% FBS and then centrifuged at 900 rpm for 30 minutes. The precipitates of Vero cells were subjected to dilution with DMEM-GGP culture media containing 10% FF and then cultured by dividing them into two 250 mL culture flasks. When they had formed a monolayer over 90% of the bottom of the flask, the Vero cells were washed 3 times with serum-free DMEM-G¼ GP culture media, after which 30 mL of fresh same culture media was placed in each flask. After culturing for 48 hours, only the culture media adapted to the Vero cells was collected and filtered through a 0.2 µm filtration device. The filtered cells were then stored at 4℃ until further analysis.
To determine the most effective serum substituent for providing optimum conditions for in vitro embryo development, hFF, SSS (99193, Irvine Scientific, Santa Ana, CA, USA), and SPS (ART3010, SAGE, Trumbull, CT, USA) were added by 10% and 20%, after which the in vitro development rate of mouse embryos was compared.
To compare the in vitro development rate of mouse embryos by storage duration of manufactured SF-VCM and to determine their available period, culture media 0, 2, 4, and 6 weeks after manufacture were utilized in the study while storing at 4℃.
To introduce controlled ovarian hyper-stimulation, 7.5 IU of pregnant mare serum gonadotropin (PMSG, Sigma) and 7.5 IU of human chorionic gonadotropin (hCG, Sigma) were injected intraperitoneally every 48 hours into female mice, after which a female and a male mouse were mated. After 15 hours, only the mice with confirmed mating plugs were utilized in the study.
To collect the 2-cell embryos, mice were sacrificed by cervical dislocation 42 to 44 hours after hCG injection, and oviducts were obtained. The oviducts were placed in a 2-well culture dish containing perfusate with 10% SSS added to Ham's F-10. The perfusate was moved through the infundibulum of the oviduct by a 1 mL syringe attached to a 31-gauge needle under a stereomicroscope. The perfusate was discharged carefully, and embryos were collected immediately after perfusion was complete. The embryos were washed multiple times in wash culture media. The 2-cell embryos were then subjected to 3 additional washings in the same culture media with in vitro culture prior to use.
For in vitro culture of 2-cell mouse embryos, 1 mL of the prepared culture media was placed in each inner well of a 2-well culture dish (3037, Falcon, Franklin Lakes, NJ, USA), and embryo development was examined while culturing in an incubator at 37℃ with CO2 for 96 hours. After 48 hours, the embryos were cultured in a new dish with fresh culture media. Ten embryos were cultured in each culture dish, and embryo development of each experimental group was examined via a microscope every 24 hours and then recorded.
This study investigated the most effective serum substituent for embryo development, the most potent concentration, and the influence of storage duration of culture media on in vitro culture of mouse embryos using serum-free conditioned culture media (SF-VCM), which was produced by culturing Vero cells in serum-free DMEM-G¼ GP with controlled levels of glucose and pyruvate. The results are shown below.
SF-VCM without any additives was the control group, and the addition of 10% and 20% hFF were classified as group I and group II, respectively. As described in Table 2, development into a morula or later stage 48 hours after culture and the development into a blastocyst or later stage 96 hours after culture was significantly more common in the groups with FF added (group I: 79.6% and 85.2%; group II: 75.9% and 83.3%, respectively) compared to the control (55.0% and 31.5%, respectively). The FF-added groups also showed significantly higher development into a hatching blastocyst or later stage 96 hours after culture, with 51.8% and 68.5% rates for group I and group II, respectively, compared to that of the control (11.7%) (P < 0.05). It was observed that the group with 20% added fluid had a significantly higher development rate than that of the 10% group.
Similar to the groups with hFF added to SF-VCM, SF-VCM without any additives was used as the control group, and the addition of 10% and 20% SSS were classified as group I and group II, respectively. The results are shown in Table 3. The development into a morula or later stage 48 hours after culture and the development into a blastocyst or later stage 96 hours after culture were significantly more common in the groups with SSS added (group I: 96.3%, 100%; group II: 100%, 100%, respectively) compared to the control (55.0% and 31.5%, respectively) (P < 0.05). Additionally, the SSS-added groups showed significantly higher development into a hatching blastocyst or later stage 96 hours after culture, with rates of 81.5% and 82.6% for group I and group II, respectively, compared to the control (11.7%) (P < 0.05). However, there was no significant difference found between the groups with 10% and 20% SSS added.
Again, SF-VCM without any additives was used as the control group, and the addition of 10% and 20% SPS were categorized into group I and group II, respectively. Table 4 represents the results of mouse embryo culture.
The development into a morula or later stage 48 hours after culture and the development into a blastocyst or later stage 96 hours after culture were significantly more common in the experimental groups utilizing SPS as the serum substituent (group I: 100%, 100%; group II: 100%, 99.1%, respectively) compared to the control (55.0% and 31.5%, respectively) (P < 0.05). Moreover, it was shown that groups with SPS added had a significantly higher rate of development into a hatching blastocyst or later stage 96 hours after culture, with rates of 94.5% and 92.7% for group I and group II, respectively, compared to that of the control (11.7%) (P < 0.05).
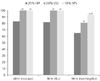
After each serum substituent (hFF, SSS, and SPS) was added to SF-VCM, the experimental groups that showed the most effective in vitro development of mouse embryos (20% hFF, 20% SSS, and 10% SPS) were selected for further analysis. The results are shown in Fig. 1. The development into a morula or later stage 48 hours after culture was significantly more common in the group with 20% SSS added (100%) and the group with 10% SPS added (100%) compared to the group with 20% hFF added (85.2%) (P < 0.05). Similarly, the development into a blastocyst or later stage 96 hours after culture was significantly more likely in the group with 20% SSS added (100%) and the group with 10% SPS added (100%) compared to the group with 20% hFF added (83.3%) (P < 0.05). In addition, significantly higher development into a hatching blastocyst or later stage 96 hours after culture was found in the group with 20% SSS added (82.6%) as well as the group with 10% SPS added (94.5%) compared to the group with 20% hFF added (68.5%). In particular, the development rate in the group with 10% SPS was found to be significantly higher than that of any group with SSS.
The authors investigated the influence of the duration of refrigeration storage of SF-VCM on mouse embryo development, and the results are described in Table 5. SF-VCM after 0 (the control group), 2, 4, and 6 weeks of storage were compared, and 10% SPS, the most effective serum substitute for in vitro development of mouse embryos, was added to each. There was no significant difference in the rate of development into a morula or later stage 48 hours after culture between the control group (96.4%) and the other groups. In contrast, the rate was significantly lower in the group with 6 weeks of storage (91.8%) compared to that of the group with 2 weeks of storage (100%) (P < 0.05). As for development into a blastocyst or later stage 96 hours after culture, both the control group (97.6%) and the group with 2 weeks of storage (98.8%) had significantly higher rates than that of the group with 6 weeks of storage (90.6%) but not compared to the group with 4 weeks of storage (95.2%). Similarly, the rate of development into a hatching blastocyst or later stage 96 hours after culture also showed significant differences in both the control group (94.1%) and the group with 2 weeks of storage (95.2%) compared to that of the group with 6 weeks of storage (84.7%) but not compared to the group with 4 weeks of storage (92.9%) (P < 0.05).
According to the meta-analysis performed by Kattal et al. [20], in vitro coculture of human in vitro fertilization and embryo transfer programs resulted in statistically significant improvements in the number of embryo blastomeres, implantation rate, and clinical pregnancy rate. Given these results, more studies are needed on in vitro coculture despite the development of many new types of culture media. Many studies reportd that coculture systems could enhance the implantation rate as well as the clinical pregnancy rate compared to conventional culture media [11,16]. Coculture has several important roles, including the secretion of embryotropic factors [21,22]; the detoxification of culture media by eliminating heavy metal ions, free radicals, or metabolic inhibitors [23,24]; and the facilitation of embryo hatching by preventing the zona hardening [25].
Vero cells, which were used as the feeder cell line of embryos in the present study, have primarily been used in the production of vaccines for a long time. Vero cells are also widely utilized as coculture cells in many studies since they are easy to store by freezing, do not contain other contaminants such as viruses, and they are stable against consistent culture [26-28]. Because somatic cells such as Vero cells attach to the culture dish or the bottom of the flask in order to proliferate instead of floating in the culture media, the addition of sera to the culture media should be considered. In the case of coculture of somatic cells with embryos, after a monolayer of somatic cells is formed, fresh culture media is provided and cultured for 1 to 2 days alone, followed by the addition of embryos in order to initiate coculture. In this case, glucose and pyruvate, which act as energy sources, should be included in the culture media.This is to prevent the floating phenomenon of attached cells, which would occur without energy sources. In addition, insufficient energy sources may be lethal for embryos. In our previous study, we focused on the production of SF-VCM using Vero cells. Since cell proliferation was somewhat restricted when sera were not added to the culture media, the present study aimed to identify the appropriate types and concentration of serum substituents that were needed for embryo development and to prevent the depletion of energy sources within culture media.
Even though an in vitro coculture system is favorable for in vitro development of embryos, it is not possible to address unfavorable influences on embryo development because the culture conditions that are affected by feeder cell lines cannot be easily controlled while coculture is being processed.
Energy sources are important in the culture media because they maximize the in vitro embryo development rate by supplying a sufficient amount of energy for early embryo development. As described in the study by Kim et al. [18], SF-VCM showed a higher development rate of mouse embryos into a hatching blastocyst or later stage compared to that of the coculture group with Vero cells, suggesting that SF-VCM could also be successfully employed in human embryo development. However, human and mouse embryos utilize energy sources in culture media differently, more studies are needed to investigate the effects of SF-VCM on in vitro culture of human embryos. Moreover, the amount of useful cytokines secreted in the coculture of manufactured serum-free culture media with Vero cells requires further study.
Generally, patient sera or cord sera are added to the in vitro embryo culture fluid as protein supplementation. In a study, the comparison between human embryo fertilization rate and in vitro development rate after adding patient's sera and synthetic Albuminar-20, revealed a similar fertilization rate in the two groups, but a higher development rate was observed in the group with Albuminar-20 added [29]. Additionally, Warnes et al. [30] found that the embryo implantation rate after freezing and thawing was remarkably lower in the culture media with human serum albumin added prior to freezing, indicating that the results after freezing and thawing differ depending on the type of protein supplementation. Desai et al. [31] observed that 10% synthetic serum substituent added to α-MEM was the protein supplementation promoting the formation of a blastocyst and could be utilized throughout all culture processes. Such results were in agreement with this study that showed effectiveness of all sera that were synthesized by adding 10% to 20% to SF-VCM. Chi et al. [32] found that hFF adding to culture media during human in vitro fertilization was a stable and potent protein supplementation. In contrast, in the present study, 20% FF added to the serum-free conditioned culture media did not show as effective of an in vitro mouse embryo development rate as other synthetic sera.
In previous studies, TCM-199 [33], DMEM [10,33], Ham's F-10 [34], and HTF [35,36] were the primary ingredients in coculture with somatic cells, and 5.5 mM of glucose was included in each of the substituents listed above as an energy source. According to Park et al. [33], when performing in vitro culture of human embryos in coculture with Vero cells utilizing TCM-199 and DMEM Vero, a noticeably higher emergence rate of the most qualified blastocysts was observed in the DMEM containing only glutamine as an energy source. Thus, DMEM culture media would be more effective for blastocyst embryo transfer in human in vitro fertilization than TCM-199 containing 5.5 mM of glucose. Similar results were exhibited in our previous study [19], which addressed the lower in vitro development rates of DMEM culture media with 5.5 mM of glucose added and coculture with Vero cells compared to those of the other experimental groups. The SF-VCM is anticipated to contain large numbers of cytokines, which facilitate a higher in vitro development rate of embryos compared to that of commercially available culture media. Such cytokines also promote an increase in the rates of embryo development, intrauterine implantation, and pregnancy. In this study, of the different serum substituents added to SF-VCM, the group with 10% SPS added resulted in significantly higher development into a hatching blastocyst or later stage 96 hours after culture (P < 0.05). In addition, the utilization of culture media up to 4 weeks resulted in no adverse effects on mouse embryo development rate. Given these results, the addition of SPS to SF-VCM is considered to be the most effective for in vitro mouse embryo culture.
In the present study, we found the types and concentration of serum substituents needed to promote mouse embryo development when added to SF-VCM. However, further studies are needed regarding the utilization of these culture media. Finally, our results should be further applied in human assisted reproductive technology to produce qualified embryos with good viability. They could then be effectively utilized to enhance implantation rate and pregnancy rate after embryo transfer.
Figures and Tables
Fig. 1
Comparison between the effects of different supplementations on in vitro development of mouse embryos cultured in SF-VCM. SF-VCM, serum free Vero cell conditioned media; hFF, human follicular fluid; SSS, serum substitute supplement; SPS, serum protein substitute; BL, blastocyst. a)P<0.05 vs. 20% hFF; b)P<0.05 v.s 20% SSS.
References
1. Betterbed B, Wright RW Jr. Development of one-cell ovine embryos in two culture media under two gas atmospheres. Theriogenology. 1985; 23:547–553.
2. Gardner DK, Lane M. Alleviation of the '2-cell block' and development to the blastocyst of CF1 mouse embryos: role of amino acids, EDTA and physical parameters. Hum Reprod. 1996; 11:2703–2712.
3. Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989; 86:679–688.
4. Brown JJ, Whittingham DG. The roles of pyruvate, lactate and glucose during preimplantation development of embryos from F1 hybrid mice in vitro. Development. 1991; 112:99–105.
5. Scott LF, Sundaram SG, Smith S. The relevance and use of mouse embryo bioassays for quality control in an assisted reproductive technology program. Fertil Steril. 1993; 60:559–568.
6. Reed ML, Jin DI, Petters RM. Glucose and inorganic phosphate inhibits rat 8-cell embryo development in vitro. Theriogenology. 1992; 37:282.
7. Takahashi Y, First NL. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology. 1992; 37:963–978.
8. Quinn P, Moinipanah R, Steinberg JM, Weathersbee PS. Successful human in vitro fertilization using a modified human tubal fluid medium lacking glucose and phosphate ions. Fertil Steril. 1995; 63:922–924.
9. Gardner DK, Lane M, Stevens J, Schoolcraft WB. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil Steril. 2001; 76:1175–1180.
10. Kim JH, Seo YS, Choi SK, Lee OS, Song HB, Kang KC, et al. The influences of different composition of glucose and pyruvate on in vitro development of mouse preimplantation embryos in medium with glutamine. Korean J Obstet Gynecol. 2004; 47:118–125.
11. Bongso A, Soon-Chye N, Sathananthan H, Lian NP, Rauff M, Ratnam S. Improved quality of human embryos when co-cultured with human ampullary cells. Hum Reprod. 1989; 4:706–713.
12. Plachot M, Antoine JM, Alvarez S, Firmin C, Pfister A, Mandelbaum J, et al. Granulosa cells improve human embryo development in vitro. Hum Reprod. 1993; 8:2133–2140.
13. Menezo YJ, Guerin JF, Czyba JC. Improvement of human early embryo development in vitro by coculture on monolayers of Vero cells. Biol Reprod. 1990; 42:301–306.
14. Cole RJ, Paul J. Properties of cultured preimplantation mouse and rabbit and cell strains developed from them. In : Wolstenholme GE, Maeve OC, editors. Ciba Foundation. Preimplantation stages of pregnancy. London: Little Brown;1965. p. 82–155.
15. Wiemer KE, Cohen J, Amborski GF, Wright G, Wiker S, Munyakazi L, et al. In-vitro development and implantation of human embryos following culture on fetal bovine uterine fibroblast cells. Hum Reprod. 1989; 4:595–600.
16. Wiemer KE, Cohen J, Wiker SR, Malter HE, Wright G, Godke RA. Coculture of human zygotes on fetal bovine uterine fibroblasts: embryonic morphology and implantation. Fertil Steril. 1989; 52:503–508.
17. Bongso A, Fong CY, Ng SC, Ratnam SS. Coculture techniques for blastocyst transfer and embryonic stem cell production. Assist Reprod Rev. 1995; 5:106–114.
18. Kim JH, Seo YS, Song HB, Yang JB, Lee KE, Lee KH. Studies on development of serum-free conditioned media using Vero cells and DMEM with controlled concentration of glucose and pyruvate. Korean J Obstet Gynecol. 2010; 53:143–151.
19. Kim JH, Lee KH, Son SK, Song HB, Kang KC. Effects of Vero cells co-culture system on the in vitro development of mouse preimplantation embryos in media with different composition of glucose and pyruvate. Korean J Obstet Gynecol. 2005; 48:1271–1281.
20. Kattal N, Cohen J, Barmat LI. Role of coculture in human in vitro fertilization: a meta-analysis. Fertil Steril. 2008; 90:1069–1076.
21. Pampfer S, Arceci RJ, Pollard JW. Role of colony stimulating factor-1 (CSF-1) and other lympho-hematopoietic growth factors in mouse pre-implantation development. Bioessays. 1991; 13:535–540.
22. Barmat LI, Worrilow KC, Paynton BV. Growth factor expression by human oviduct and buffalo rat liver coculture cells. Fertil Steril. 1997; 67:775–779.
23. Loutradis D, John D, Kiessling AA. Hypoxanthine causes a 2-cell block in random-bred mouse embryos. Biol Reprod. 1987; 37:311–316.
24. Fukui Y, McGowan LT, James RW, Pugh PA, Tervit HR. Factors affecting the in-vitro development to blastocysts of bovine oocytes matured and fertilized in vitro. J Reprod Fertil. 1991; 92:125–131.
25. Van Blerkom J. Development of human embryos to the hatched blastocyst stage in the presence or absence of a monolayer of Vero cells. Hum Reprod. 1993; 8:1525–1539.
26. Magli MC, Gianaroli L, Ferraretti AP, Fortini D, Fiorentino A, D'Errico A. Human embryo co-culture: results of a randomized prospective study. Int J Fertil Menopausal Stud. 1995; 40:254–259.
27. Kaufman RA, Menezo Y, Hazout A, Nicollet B, DuMont M, Servy EJ. Cocultured blastocyst cryopreservation: experience of more than 500 transfer cycles. Fertil Steril. 1995; 64:1125–1129.
28. Turner K, Lenton EA. The influence of Vero cell culture on human embryo development and chorionic gonadotrophin production in vitro. Hum Reprod. 1996; 11:1966–1974.
29. Staessen C, Van den Abbeel E, Carle M, Khan I, Devroey P, Van Steirteghem AC. Comparison between human serum and Albuminar-20 (TM) supplement for in-vitro fertilization. Hum Reprod. 1990; 5:336–341.
30. Warnes GM, Payne D, Jeffrey R, Hourigan L, Kirby C, Kerin J, et al. Reduced pregnancy rates following the transfer of human embryos frozen or thawed in culture media supplemented with normal serum albumin. Hum Reprod. 1997; 12:1525–1530.
31. Desai N, Kinzer D, Loeb A, Goldfarb J. Use of synthetic serum substitute and alpha-minimum essential medium for the extended culture of human embryos to the blastocyst stage. Hum Reprod. 1997; 12:328–335.
32. Chi HJ, Kim DH, Koo JJ, Chang SS. The suitability and efficiency of human follicular fluid as a protein supplement in human in vitro fertilization programs. Fertil Steril. 1998; 70:871–877.
33. Park KS, Choi IK, Lee JS, Song HB. The effects of glutamine on blastulation of human embryos on Vero cells in vitro. Korean J Fertil Steril. 1998; 25:65–70.
34. Sakkas D, Jaquenoud N, Leppens G, Campana A. Comparison of results after in vitro fertilized human embryos are cultured in routine medium and in coculture on Vero cells: a randomized study. Fertil Steril. 1994; 61:521–525.
35. Lee KS, Ko HG, Shin BS, Lee YA, Kim SW, Na YJ. The effect of coculture with human oviductal cells on in vitro development of ICR mouse embryo. Korean J Obstet Gynecol. 2000; 43:1029–1036.
36. Lee KS, Kim SW, Na YJ, Lee YA, Kim HJ, Jang SK. Role of leukemia inhibitory factor in the effect of co-culture on preimplantation embryo develpement. Korean J Obstet Gynecol. 2000; 43:1216–1222.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download