Abstract
Objective
Nuchal translucency (NT) is the most powerful screening tool for Down syndrome and congenital cardiac anomaly, therefore strict guidelines were established to get accurate NT values. However, to stick to the guideline in all pregnant women is time-consuming and superfluous in majority of low risk population. We undertook this study to investigate whether the simplified protocol enables to select low risk group and is effective in them even if we skip the suggested NT measurement.
Methods
NT and crown-rump length (CRL) were measured prospectively. First, CRL was measured in the ordinary view that was mid-sagittal section of fetus in neutral position, and NT was measured at the same frozen screen (first measured value, 1MV). Then, NT was measured again according to the Fetal Medicine Foundation (FMF) guideline (second measured value, 2MV).
Results
There was good correlation between 1MV and 2MV in each case (r = 0.83, P < 0.001). All of the NT values over the 95th percentile in 2MV also belonged to over the 95th percentile in 1MV. NT value of 2 mm in 1MV could be used as a cut-off to obtain over the 95th percentile 2MV by receiver operating characteristic curve (sensitivity 100%, specificity 80.5%). The proportion of 1MV ≥ 2 mm was only 23.8% of all cases, namely we had only to measure 2MV in 23.8% patients. Every 95th percentile or more 2MV could be detected with this simplified protocol.
Nuchal translucency (NT) measurement is the most powerful screening tool for Down syndrome and congenital malformations, especially cardiac anomaly [1-11]. The Fetal Medicine Foundation (FMF) established the strict guideline for NT measurement. The guideline is used worldwide and requires a certification and annual re-audit for NT measurement. However, to stick to the guideline seems time-consuming and superfluous in some pregnant women whose NT is definitely thin at a glance. We undertook this study to simplify the NT measurement and to investigate whether the simplified protocol is effective in low risk population even if we skip the suggested NT measurement according to the established guideline.
A prospective study was conducted. Transabdominal ultrasound examination was performed as a part of routine prenatal checks at 11+0 to 13+6 weeks of gestation and the fetal crown-rump length was 45 to 84 mm. The gestational age was basically calculated from the first day of the last menstrual period and was confirmed by crown-rump length (CRL) measurement [12]. If there was some discrepancy of more than 7 days between gestational ages by menstrual calculation and by ultrasound estimation, the latter was used. In cases of multiple pregnancies, the same cut-off value was applied as that of singleton pregnancy [3,13]. All measurements were obtained by one skilled examiner (S.M.K.) with one ultrasound unit, Accuvix XQ (Medison, Seoul, Korea).
The Institutional Review Board of our institution (Seoul National University Hospital, Seoul, Korea) has approved the collection and utilization of clinical and sonographic data for the research purposes.
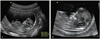
First, CRL was measured in the ordinary view that was mid-sagittal section of fetus in neutral position, and NT was measured at the same frozen screen (first measured value, 1MV) (Fig. 1A). Then, NT was measured three times again according to the FMF guideline (second measured value, 2MV) (Fig. 1B) and out of three measurements, the maximal value was selected. The measurement of 2MV should be met the following criteria [14]; 1) mid-sagittal section of the fetus with neutral position; 2) magnification so that only fetal head and upper thorax included in the image; 3) maximal thickness of the subcutaneous translucency between the skin and the soft tissue overlying the cervical spine; 4) distinction between fetal skin and amnion; 5) the crossbar of the caliper on the white line of the border and not in the nuchal fluid.
We use the standard values of the NT with CRL distribution in Korean population [15].
Differences between the values of 1MV and 2MV were tested by means of Wilcoxon signed ranks test and associations were described by Spearman's correlation coefficient. Receiver operator characteristic (ROC) curve was used to select the cut-off value of the 1MV that needs to be measured according to the FMF criteria for detection of the 95th percentile or more 2MV. A probability value of <0.05 was considered statistically significant.
Three hundred fifteen fetuses were enrolled. Sixty-seven twin fetuses and seven triplet fetuses were included. In seven patients, NT was so thin that cursors could not be situated at their exact points in the 1MV, and their 2MV values were also very thin, under the 1.0 mm.
There was a good correlation between the 1MV and the 2MV (r = 0.83 by spearman's rho, P<0.001). There was no statistically significant difference between the 1MV and the 2MV by Wilcoxon signed ranks test. All of the NT values over the 95th percentile in the 2MV also belonged to over the 95th percentile in the 1MV.
ROC curve was conducted to examine the relationship between the 1MV and over the 95th percentile 2MV (area under curve, 0.98; P<0.001) (Fig. 2). NT value of 2 mm in the 1MV could be used as a cut-off to obtain the 95th percentile or more 2MV (sensitivity of 100%, specificity of 80.5%, positive predictive value 22.7%, negative predictive value 100%). The proportion of the 1MV ≥2 mm was only 23.8% of all cases, in other words, we had only to measure the 2MV in 23.8% patients. Every 95th percentile or more 2MV could be detected with this simplified protocol. There was no missed case with abnormal NT.
In this study, with simplified protocol of NT measurement, we tried to identify low risk women who did not have to undergo the NT measurement by the stringent existing guideline. We have high regard for the established guideline of NT measurement by the FMF, but there was some doubt whether the guideline had to be applied to all pregnant women including low risk population with very thin NT at a glance. We would like to suggest a protocol that identifies low risk women who do not have to measure their fetal NT according to the established guideline and can simplify NT measurement eventually. NT measurement is not diagnostic of any specific disorder with its absolute value, rather, it is just screening process identifies pregnant women who have sufficient risks for Down syndrome or cardiac anomalies to warrant genetic counseling and additional diagnostic tests, such as chorionic villous sampling, amniocentesis, or fetal echocardiography [16]. Our suggestion could be very useful considering the purpose of NT measurement is just to select cases with over the 95th percentile NT values for their CRL.
There is a similar screening test like our simplified protocol in obstetrical field. A 50-g oral glucose tolerance test (OGTT) is a similar example like a simplified protocol. A woman does not have to undergo a cumbersome 100-g OGTT for confirmed diagnosis if her glucose level is under the cut-off in a simple 50-g OGTT. On the basis of our data, 23.8% of pregnant women showed NT measurement ≥2.0 mm at 1MV and almost 3/4 pregnant women can circumvent original NT measurement. It results in decreasing time of examination and medical cost.
The exact value of NT cannot be obtained in some women by our simplified protocol because women whose fetuses have thin NT below 2 mm at the 1MV may skip the measurement according to the established guideline. This may be inappropriate in the combined first trimester screening tests that analyze the results out of maternal serum markers and NT values, both. However, in the centers where the combined first trimester screening tests are not available, our protocol can be useful for saving time and effort in NT measurement. Actually, there are many centers where only NT measurement is used as the first trimester screening test because of various reasons. Especially, the combined first trimester screening test is not covered by national health insurance in Korea.
Considering the role of NT measurement as a screening tool, false positive results can be accepted more generously than false negative results. Our protocol is safe method in this sense. The differences between simplified protocol and the established guideline are proper magnification and identification of fetal skin from amniotic membrane. Neutral position and mid-sagittal section of fetus are also required in our simplified protocol to obtain an exact CRL. Generally, measurement values decrease with increasing image size [17-19]. Therefore, the second MV, in other words, exact NT value measured by the established guideline will have possibility to be somewhat smaller than the 1MV. There were several cases with the larger 2MV than the 1MV in our study. However, most of them had very thin NT measurements. It may be caused by incorrect placement of caliper in the 1MV because the NT is too thin to place the caliper on the exact position without magnification. And if fetal skin is confused with amniotic membrane in the 1MV, the 2MV that measured properly by the established guideline will be further smaller than the 1MV. Therefore, there is little risk missing the abnormal NT values by our simplified protocol. NT measurement of this study was conducted by one skilled examiner. CRL should be measured in the midsagittal plane and it is not always easy to a novice at obstetric ultrasonography. To apply simplified NT measurement, one should be cautious of taking a correct midsagittal plane of CRL.
In conclusion, we suggest a simplified protocol that if the NT is less than 2 mm at ordinary CRL view, the formal measurement according to the established guideline may be skipped. It can be effective for saving time and effort for NT measurements especially in some clinical settings such as multiple pregnancies or where the combined first trimester screening test can't be used.
Figures and Tables
Notes
References
1. Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ. 1992; 304:867–869.
2. Pandya PP, Snijders RJ, Johnson SP, De Lourdes Brizot M, Nicolaides KH. Screening for fetal trisomies by maternal age and fetal nuchal translucency thickness at 10 to 14 weeks of gestation. Br J Obstet Gynaecol. 1995; 102:957–962.
3. Sebire NJ, Snijders RJ, Hughes K, Sepulveda W, Nicolaides KH. Screening for trisomy 21 in twin pregnancies by maternal age and fetal nuchal translucency thickness at 10-14 weeks of gestation. Br J Obstet Gynaecol. 1996; 103:999–1003.
4. Hyett JA, Moscoso G, Nicolaides KH. First-trimester nuchal translucency and cardiac septal defects in fetuses with trisomy 21. Am J Obstet Gynecol. 1995; 172:1411–1413.
5. Galindo A, Comas C, Martnez JM, Gutirrez-Larraya F, Carrera JM, Puerto B, et al. Cardiac defects in chromosomally normal fetuses with increased nuchal translucency at 10-14 weeks of gestation. J Matern Fetal Neonatal Med. 2003; 13:163–170.
6. Makrydimas G, Sotiriadis A, Huggon IC, Simpson J, Sharland G, Carvalho JS, et al. Nuchal translucency and fetal cardiac defects: a pooled analysis of major fetal echocardiography centers. Am J Obstet Gynecol. 2005; 192:89–95.
7. Haak MC, Bartelings MM, Gittenberger-De Groot AC, Van Vugt JM. Cardiac malformations in first-trimester fetuses with increased nuchal translucency: ultrasound diagnosis and postmortem morphology. Ultrasound Obstet Gynecol. 2002; 20:14–21.
8. Sebire NJ, Snijders RJ, Davenport M, Greenough A, Nicolaides KH. Fetal nuchal translucency thickness at 10-14 weeks' gestation and congenital diaphragmatic hernia. Obstet Gynecol. 1997; 90:943–946.
9. Kagan K, Avgidou K, Molina F, Gajewska K, Nicolaides K. Relation between increased fetal nuchal translucency thickness and chromosomal defects. Obstet Gynecol. 2006; 107:6–10.
10. Souka AP, Snijders RJ, Novakov A, Soares W, Nicolaides KH. Defects and syndromes in chromosomally normal fetuses with increased nuchal translucency thickness at 10-14 weeks of gestation. Ultrasound Obstet Gynecol. 1998; 11:391–400.
11. Brambati B, Cislaghi C, Tului L, Alberti E, Amidani M, Colombo U, et al. First-trimester Down's syndrome screening using nuchal translucency: a prospective study in patients undergoing chorionic villus sampling. Ultrasound Obstet Gynecol. 1995; 5:9–14.
12. Hadlock FP, Shah YP, Kanon DJ, Lindsey JV. Fetal crown-rump length: reevaluation of relation to menstrual age (5-18 weeks) with high-resolution real-time US. Radiology. 1992; 182:501–505.
13. Maslovitz S, Yaron Y, Fait G, Gull I, Wolman I, Jaffa A, et al. Feasibility of nuchal translucency in triplet pregnancies. J Ultrasound Med. 2004; 23:501–504.
14. Fetal Medicine Foundation. Online education: the 11-13 weeks scan [Internet]. London: Fetal Medicine Foundation;2004. cited 2010 Sep 12. Available from: http://www.fetalmedicine.com.
15. Chung JH, Yang JH, Song MJ, Cho JY, Lee YH, Park SY, et al. The distribution of fetal nuchal translucency thickness in normal Korean fetuses. J Korean Med Sci. 2004; 19:32–36.
16. Palomaki GE, Lee JE, Canick JA, McDowell GA, Donnenfeld AE. ACMG Laboratory Quality Assurance Committee. Technical standards and guidelines: prenatal screening for Down syndrome that includes first-trimester biochemistry and/or ultrasound measurements. Genet Med. 2009; 11:669–681.
17. Teoh M, Meagher SE, Choong S, Shekleton P, Wallace EM. The effect of image size on screen-positive rates for nuchal translucency screening. BJOG. 2006; 113:479–481.
18. Hsu JJ, Chiang CH, Hsieh CC, Hsieh TT. The influence of image magnification in first-trimester screening for Down syndrome by fetal nuchal translucency in Asians. Prenat Diagn. 2004; 24:1007–1012.
19. Edwards A, Mulvey S, Wallace EM. The effect of image size on nuchal translucency measurement. Prenat Diagn. 2003; 23:284–286.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download