Abstract
Objective
Postpartum hemorrhage is most common cause of maternal mortality. Active management of third stage of labor minimizes the risk of postpartum hemorrhage. To compare the effect of methylergonovine and 15-methyl prostaglandin F2α (15-methyl PGF2α) in active management of third stage of labor.
Methods
A randomized open labelled parallel study with 50 women in normal labor, 25 in each group were included. The drugs methylergonovine (0.2 mg) intravenous and 15-methyl PGF2α (250 µg) intramuscular were administered at the time of delivery of anterior shoulder. Main outcomes measured were amount of blood loss during the first four hours of delivery and objective measurement of hemoglobin and hematocrit levels before delivery and third day postpartum.
Results
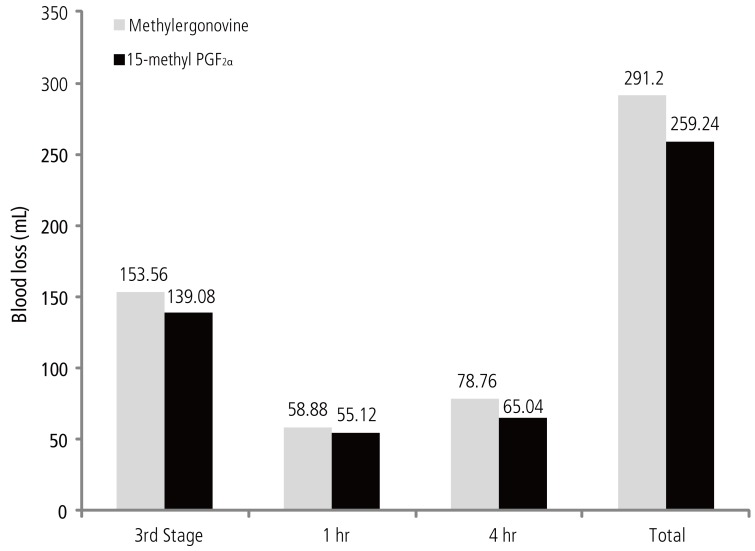
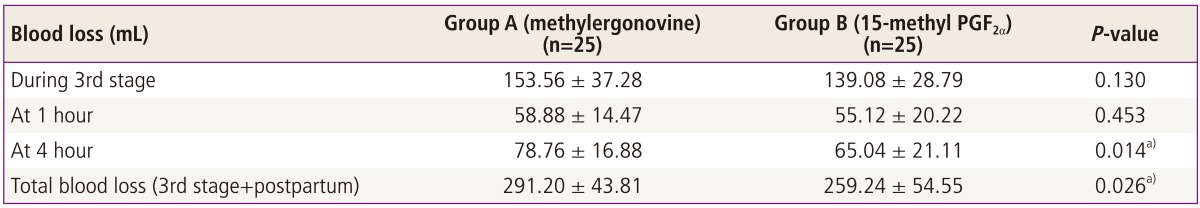
There was no statistically significant difference in the blood loss between the two groups at delivery (P = 0.130), at 1 hour of delivery (P = 0.453). The blood loss with 15-methyl PGF2α was significantly less as compared to that of blood loss with methylergonovine at four hours of delivery (P = 0.014) and the total, i.e., during first four hours, amount of blood loss was significantly less with 15-methyl PGF2α (P = 0.026). There was no statistically significant difference in the hemoglobin and hematocrit levels measured predelivery and postpartum third day between both the drugs.
Postpartum hemorrhage (PPH) is one of the most common causes of maternal mortality [1]. In the developing world, it accounts for 30% of such deaths in areas where maternal mortality is high, and less than 10% where it is low, e.g., in developed countries [2]. Life threatening obstetric hemorrhage occurs in approximately 1 per 1,000 deliveries [3].
Two types have been described, i.e., primary PPH or early PPH occurring within first 24 hours and secondary PPH or late PPH that occur more than 24 hours after delivery.
The most common cause of PPH is uterine atony and it accounts for 80% to 85% cases. In many women, its subsequent occurrence can be suspected before delivery. Over-distended uterus is more prone to hypotony such as women with large fetus, multiple fetuses and hydramnios. Labor with vigorous activity or with barely effective contractions increases the risk of uterine atony. Similarly, labor initiated or augmented with oxytocin is more likely to be followed by atony and hemorrhage [4]. Myometrial contractility is integral to the delivery of placenta and the arrest of the potential subsequent hemorrhage. There is an important contribution of the hormones for this physiological process [5].
Methylergonovine (methylergometrine) is a semi-synthetic ergot alkaloid derivative, first drug to be used for the active management of third stage of labor. It acts as a partial agonist at α-adrenergic (α1) and serotonergic (5-HT2) receptors. It causes constriction of uterine vascular smooth muscle. The sensitivity of the uterus increases to α1 receptor with progress in pregnancy. At term, the pregnant uterus is most sensitive to the drug. It produces alternate contraction and relaxation at low doses but induces powerful and prolonged contracture at high doses, which is the basis of its use in PPH [6].
Its oral absorption is rapid in contrast to other ergot alkaloids [7] and reaches peak plasma concentration within 60 to 90 minutes. Uterotonic effect in postpartum women is seen within 10 minutes of oral administration and immediately after intravenous administration. It is used alone or in combination with oxytocin in the prevention and treatment of PPH [8]. The intensity of pressor response is enhanced when the blood pressure is already elevated, therefore its use is contraindicated in women with hypertension.
Prostaglandins, both prostaglandin E1 and prostaglandin F2α (PGF2α ), are considered to be the physiological stimuli for myometrial contractility [8]. 15-methyl PGF2α is a methylated derivative with long duration of action as compared to PGF2α. It acts on FP type of prostaglandin receptors. The sensitivity of pregnant uterus to 15-methyl PGF2α increases with progression in pregnancy. It causes dose-dependent increase in uterine tone, as well as frequency and amplitude of uterine contractions [9]. It is a highly effective drug in the management of massive bleeding [10]. Clinical studies have shown success rates of 80% to 90% in the treatment of postpartum hemorrhage refractory to both oxytocin and ergot alkaloids alkaloids [11].
The study aimed at comparing the efficacy and tolerability of both methylergonovine, 15-methyl PGF2α in the active management of third stage of labor and to compare the efficacy of the above two drugs. The effect of these drugs was studied on the amount of blood loss during third stage of labor, at 1 hour and 4 hours after delivery. The percentage change (% fall) in mean hemoglobin and hematocrit was studied before delivery and on third day postpartum.
A total of 50 women in labor were included in the study after obtaining informed consent. It was an open label randomized parallel comparative study, approved by institutional ethics committee of a tertiary medical college and hospital. The study was conducted in the labor room of Department of Obstetrics and Gynaecology. All of the women had routine antenatal investigations including hemoglobin estimation, urinanlysis. They were allotted to receive either methylergonovine 200 µg intravenously (group A) or 15-methyl PGF2α 250 µg intramuscularly (group B) at the time of delivery of the anterior shoulder of the baby, by placing of computer generated random allocations into consecutively numbered identical white colored sealed envelopes. At the time of delivery itself, the envelopes were opened. The various inclusion and exclusion criteria were:
Age, 18-40 years; gestation ≥34 weeks; normal vaginal delivery; gravida 1-5; cephalic presentation & longitudinal lie.
Previous Cesarean section; intrauterine death, multifetal pregnancy; PPH in previous pregnancy; hydramnios, induced labor; history of allergic reactions and coagulation defects.
All women were monitored during the third stage of labor, till third day postpartum. Records were kept about the blood loss, duration of third stage (by noting the time between the delivery of the baby and delivery of the placenta.
The blood loss during the first four hours of delivery was measured objectively, amount of blood loss was assessed at the time of delivery, at one hour and four hours post delivery. It was estimated by weighing the blood clots and used pads. During delivery the blood was collected in kidney tray (350 mL) and measured whereas the blood loss at 1 hour and 4 hours was assessed by weighing the used pads during that period. These were previously autoclaved pads which were weighed on digital pediatric weighing machine. After use, these were put in an air-tight polybag and weighed again. The weight of the polybag was subtracted.
Loss of blood was calculated as: blood loss (in g) in first four hours postpartum=weight of used pads (g)-weight of unused pads (g) (1 g=1 mL).
Total blood loss (mL)=blood loss during delivery (mL)+blood loss in first four hours postpartum (mL).
On third day postpartum the blood sample was again taken for hemoglobin and hematocrit measurement. The sample was sent to pathology department for objective measurement. The percentage change of hemoglobin and hematocrit levels predelivery and postpartum third day levels was measured.
The data obtained was entered and analyzed by applying Student's t-test and chi-square test. Women were also monitored for adverse effects such as nausea, diarrhea, vomiting and hypertension.
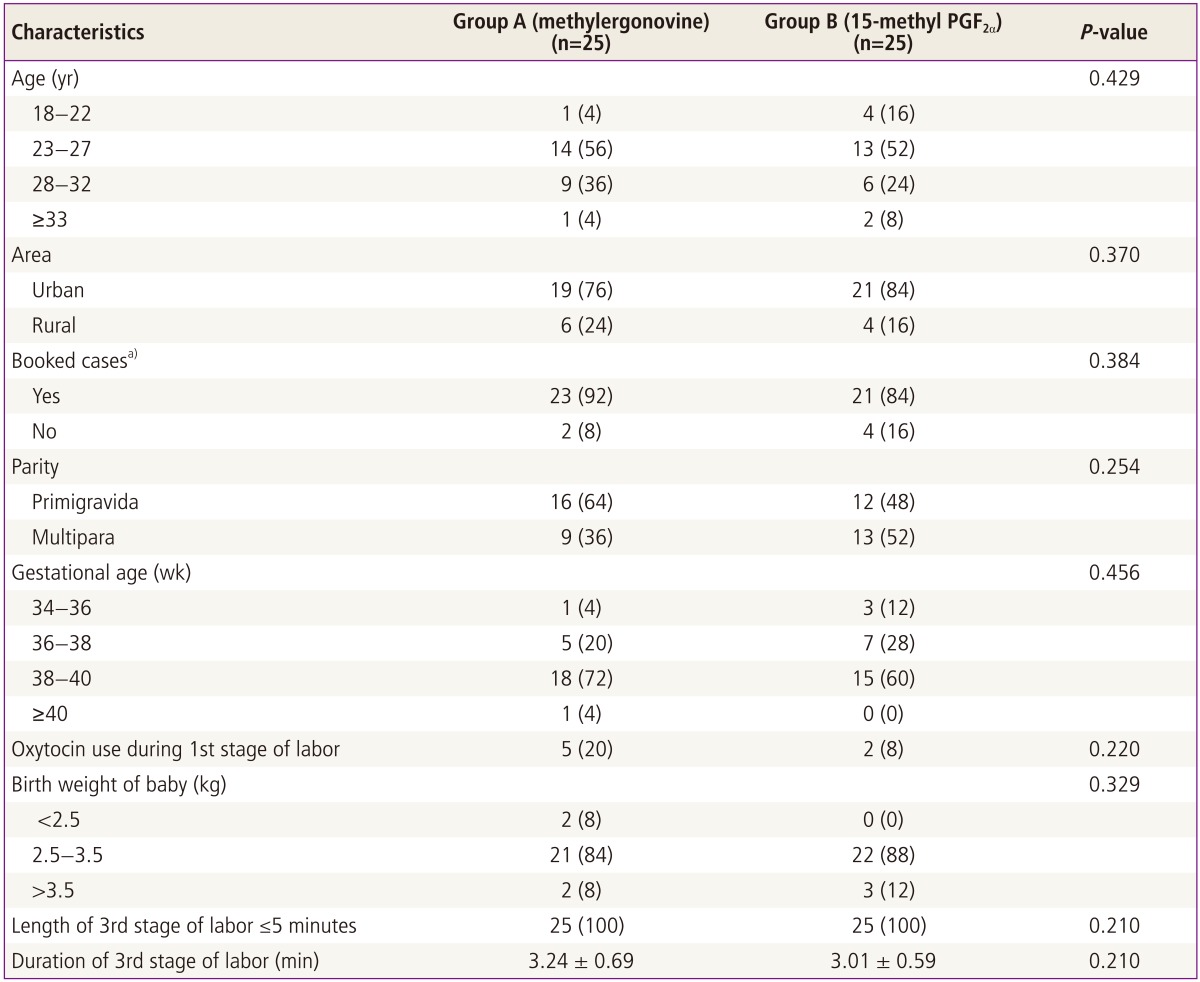
All the women were in the age group of 18 to 38 years. Most of women were in the age group of 23 to 27 years in both the groups. Most of the women had their antenatal check-ups in both the groups, i.e., booked cases. The parity and gestational age distribution in both the groups are shown in Table 1.
Most of the nullipara in both the group were given oxytocics during the first stage of labor for induction. Two multipara were given oxytocin in group B to induce sustained contractions. Others had a spontaneous labor and normal vaginal delivery.
The mean duration of third stage of labor in methylergonovine was 3.24±0.69 minutes and in 15-methyl PGF2α, it was 3.01±0.59 minutes. There was no case of retained placenta and no extra effort was required for placental removal in any of the cases. Comparison between two groups showed no statistically significant difference in the duration of third stage of labor with either drug (unpaired t =1.26, P =0.213) (Table 1).
The study compared the total amount of blood loss (during third stage, at 1 hour and at 4 hours after delivery) between the two groups. None of the woman developed PPH. The amount of blood loss comparison between the two groups showed following results (Table 2).
Therefore, the mean blood loss from third stage of labor till first 4 hours after delivery in the methylergonovine group was 291.20 ± 43.81 mL, whereas in 15-methyl PGF2α group, it was 259.24 ± 54.55 mL. The inter-group comparison showed a statistically significant difference in the total amount of blood loss (unpaired t =2.28, P = 0.026) (mean blood loss during first 4 hours) (Fig. 1).
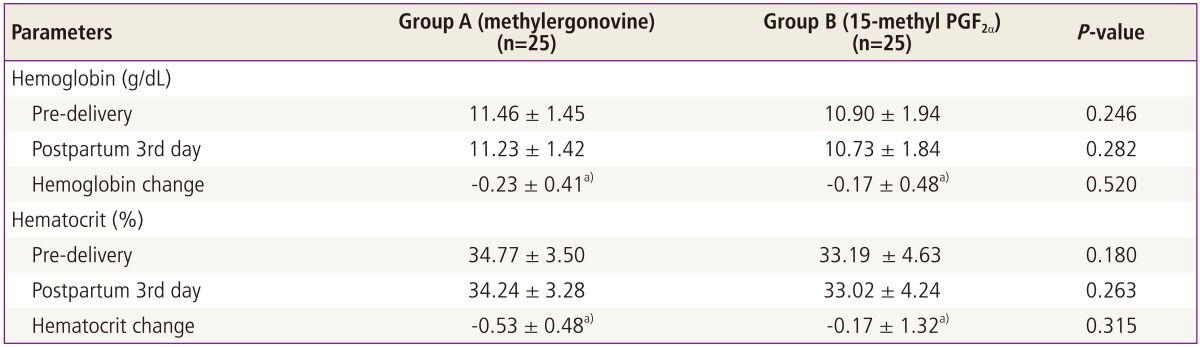
The difference of hemoglobin change (i.e., predilevery and 3rd day postpartum) between group A and B was insignificant (unpaired t = 0.65, P = 0.520). Similarly the difference in hematocrit between the two groups measured both pre-delivery and post-delivery (unpaired t = 1.01, P = 0.315) was insignificant (Table 3).
Adverse effects noted in methylergonovine group (A) was a single episode of vomiting (4%) whereas in the 15-methyl PGF2α group (B), 1 woman had nausea and vomiting (4%), 2 women (8%) had diarrhea and 1 had fever (4%).
PPH is one of the leading causes of maternal mortality. It is excessive blood loss, i.e., more than 500 mLin vaginal delivery and more than 1,000 mL in Cesarean section.
The various predisposing factors for PPH includes a history of postpartal hemorrhage in previous pregnancy, prolonged, augmented or rapid labor, pre-eclampsia, operative delivery, chorioamnionitis, trauma to the genital tract, i.e., large episiotomy, laceration of the perineum or an overdistended uterus due to macrosomia, twins, or hydramnios and coagulation defects. Many of these risk factors can be identified during prenatal care or in early labor so that, ideally, women are referred to a hospital-based facility where prophylaxis and treatment are available [4].
Physiological adaptation of the cardiovascular system in pregnancy results in 48% increase in plasma volume and 17% increase in red cell mass [12]. There is protective hemodilution resulting in fall in hemoglobin, hematocrit, red cell count but maintains mean corpuscular volume and mean corpuscular hemoglobin concentration. Circulatory blood volume rises by 37%, which provides adequate placental perfusion and a compensatory reserve for acute blood loss during delivery. It has been found that visual estimation of blood loss at vaginal and abdominal delivery remains inaccurate [5]. Most of the times it is half of what the actual loss is. Loss of blood should be measured objectively and not subjectively. A standardized way should be followed rather than by clinical estimation, which underestimates blood loss especially when it is more than 1,000 mL [13].
With the progression of pregnancy, the uterus becomes sensitive to the drugs, e.g., methylergonovine, oxytocin and prostaglandins.
Many studies have been conducted till now and shown that active management of third stage of labor is superior to expectant management in terms of blood loss, duration of third stage and other serious complications. The active management includes use of prophylactic oxytocics, cord clamping before the delivery of placenta and cord traction. Cochrane Database Review [14]of many studies has revealed that active management is associated with significantly lesser blood loss during the third stage of labor.
Till date studies have used single dose (0.2 mg or 0.25 mg) of methylergonovine either intramuscularly or intravenously for the prophylactic management and all these studies have shown it to be effective to control blood loss during third stage and also to reduce the duration of third stage of labor significantly. Similarly, single dose of 15-methyl PGF2α (125 µg IM or 250 µg IM) is effective in controlling blood loss during third stage of labor.
In this study the blood loss measured objectively was significantly less with 15-methyl PGF2α as compared to that of methylergonovine. The mean duration of third stage of labor was found to be less than 5 minutes in both the groups none of the women in either group had complications of third stage of labor, i.e., established PPH, need for another uterotonic, retained placenta, etc. As 15-methyl PGF2α has a disadvantage of high cost, therefore it is not feasible to be used in all cases for prophylactic management of third stage of labor especially so in developing countries like India. Both methylergonovine and 15-methyl PGF2α have good efficacy when used as a prophylactic measure for postpartum hemorrhage. Although the decrease in blood loss is significantly more with 15-methyl PGF2α but the cost and adverse drug reaction profile is better for methylergonovine.
To conclude both the drugs were effective in hemostasis during the active management of third stage of labor. None of the women had blood loss more than 500 mL in either group. 15-methyl PGF2α was found to be more efficacious in controlling the amount of blood loss during first four hours of delivery as compared to methylergonovine in the study. But the adverse drug reactions associated with 15-methyl PGF2α are more as compared to that of methylergonovine. Also, the cost involved with 15-methyl PGF2α is much higher. Therefore 15-methyl PGF2α can be recommended in cases where methylergonovine is contraindicated, and in cases refractory to other uterotonic agents. On the basis of the present study, it does not appear to be capable of replacing methylergonovine as the drug of first choice in the active management of third stage of labor.
References
1. Dildy GA 3rd. Postpartum hemorrhage: new management options. Clin Obstet Gynecol. 2002; 45:330–344. PMID: 12048393.

2. El-Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, Walder J, et al. The misoprostol third stage of labor study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG. 2000; 107:1104–1110. PMID: 11002953.
3. Drife J. Management of primary postpartum haemorrhage. Br J Obstet Gynaecol. 1997; 104:275–277. PMID: 9091001.

4. Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, Wenstrom KD. Obstetrical hemorrhage. In : Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, Wenstrom KD, editors. William's obstetrics. 22nd ed. New York: McGraw-Hill;2005. p. 809–854.
5. Bose P, Regan F, Paterson-Brown S. Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. BJOG. 2006; 113:919–924. PMID: 16907938.

6. Sanders-Bush E, Mayer SE. 5-Hydroxytryptamine (serotonin): receptor agonist and antagonist. In : Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gillman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill;2005. p. 297–315.
7. Tripathi KD. 5-Hydroxytryptamine, its antagonists and drug therapy of migraine. In : Tripathi KD, editor. Essential of medical pharmacology. 6th ed. New Delhi: Jaypee brothers;2008. p. 162–172.
8. Jaiswal N, Joshi V, Sapre S, Olyai R. Comparative study between per rectal misoprostol and IM methergin for prophylaxis of PPH. Obstet Gynecol Today. 2006; 3:160–162.
9. Smyth EM, Burke A, FitzGerald GA. Lipid-derived autacoids: eicosanoids and platelet-activating factor. In : Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gillman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill;2005. p. 653–670.
10. Schuurmans N, MacKinnon C, Lane C, Etches D. Prevention and management of postpartum haemorrhage. SOGC Clinical Practice Guidelines. J Soc Obstet Gynaecol Can. 2000; 22:271–281.
11. Schellenberg JC. Primary postpartum haemorrhage (PPH) [Internet]. Versoix, Switzerland: Geneva Foundation for Medical Education and Research;2013. cited 2008 May 24. Available from: http://www.gfmer.ch/Endo/Lectures_09/primary_postpartum_haemorrhage.htm.
12. Hofmeyr GJ, Mohlala BK. Hypovolaemic shock. Best Pract Res Clin Obstet Gynaecol. 2001; 15:645–662. PMID: 11478820.

13. Gulmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labor. Lancet. 2001; 358:689–695. PMID: 11551574.
14. Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labor. Cochrane Database Syst Rev. 2000; (3):CD000007.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download