Abstract
Mature cystic teratomas of the ovary (MCT) are usually observed in women of reproductive age with the most dreadful complication being malignant transformation which occurs in approximately 1% to 3% of MCTs. In this case report, we present a patient with squamous cell carcinoma which developed from a MCT during pregnancy. The patient was treated conservatively without adjuvant chemotherapy and was followed without evidence of disease for more than 60 months using conventional tools as well as positron emission tomography-computed tomography following the initial surgery. We report this case along with the review of literature.
The incidence of adnexal masses during pregnancy is 1% to 9% [1]. Mature cystic teratomas (MCT) are common during pregnancy with the most dreadful complication being malignant transformation which occurs in approximately 1% to 3% of MCTs [2]. In comparison with benign MCT, malignant transformation occurs in the older population with a mean age of 45 to 60 years [3]. The prevalent malignant evolution is a squamous cell carcinoma (SCC) accounting for 63.7% to 88.9% of all malignancies originating from dermoid tumours [2]. There are no established standard preoperative diagnostic, surgical or postoperative procedures due to the low incidence of cases [4,5].
A case of SCC which developed from an MCT in a 30-year-old woman during pregnancy is presented and issues regarding the diagnosis and management of this rare complication are discussed.
A 30-year nulliparous woman was referred to our clinic for a tumor in the left lower quadrant at 8+5 weeks of gestation. Ultrasonography revealed a tumour of the left ovary (about 18 cm) (Fig. 1A, B).
An 18 cm solid and cystic left ovarian mass with a smooth surface and two small right ovarian cysts were detected resulting in a laparotomy at 13 weeks of gestation and left salpingo-oophorectomy and right ovarian cystectomy (Fig. 1C). The report of the frozen section from both tissues revealed MCT. The permanent histology report showed a 17×13×10 cm, 1,029 g MCT. In addition, a well-differentiated invasive SCC was detected arising from the MCT of the left ovary and confined within the MCT of the ovary which did not involve the surface of the ovary (Fig. 2).
SCC antigen levels were assayed following the report of SCC and were 0.37 ng/mL. There was no evidence of metastasis to the brain, chest, abdomen and pelvis via MRI. The patient opted for continuation of the pregnancy and delivered a healthy female 3.3 kg at term of gestation through vaginal route 6 months later following the operation. After delivery, positron emission tomography-computed tomography (PET-CT) was performed revealing no significant hypermetabolic lesions suggestive of a lack of recurrence or metastasis. However, there was diffuse increased fluorodeoxyglucose (FDG) uptake in both breasts during breast feeding. One year following the operation, the patient presented with complaints of headache but a brain angio-CT was normal. Two years after her first delivery, the patient underwent Cesarean section for a twin delivery: 2 females weighing 2.74 kg each. The tissue sampled during the Cesarean section showed no pathologic finding. Two and a half years following the first operation, there was an abnormal hypermetabolic lesion in the right ovary on PET-CT suggesting a physiologic rather than pathologic uptake. However, there was no demonstration of any recurred mass or abnormal lymphadenopathy in the pelvis on follow-up magnetic resonance imaging (MRI). The PET-CT performed one month later revealed no significant hypermetabolic lesions to suggest recurrence or metastasis. However, four years after the initial operation, there was an abnormal hypermetabolic lesion in the right ovary and endometrium. Follow-up MRI revealed a small sized uterine myoma with multiple follicular cysts in the right ovary. During the follow-up period, every cyst as viewed on sonography had faded out. During the five years postoperation, 10 measurements of the SCC level had been performed and these values were within normal limits. The patient was taking care of her 3 kids following the initial operation for more than 60 months without any issues.
The management of persistent adnexal masses during pregnancy is controversial. Following cervical cancer, ovarian cancer is the second most frequent gynaecological neoplasm complicating pregnancy [6]. Leiserowitz et al. [7] reported that ovarian cancers diagnosed during pregnancy were mostly borderline lesions or germ cell tumours and the overall prognosis is highly favorable.
SCC is rarely diagnosed pre- or intraoperatively due to its rare incidence and generally occurs after 40 years of age with the mean age ranging from 45 to 60 years. Preoperative distinction between MCT and SCC is extremely difficult due to the rarity of malignant transformation and the complex contents of MCT. Studies examining the clinical viability of tumour markers such as CA-125, CEA, CA-19-9, and SCC antigen have proven inconclusive [8]. Though MCT presents with a wide range of size, malignant transformation correlates with increasing dimensions. It has been recommended that a tumour size greater than 10 cm should prompt suspicion [9]. In our case, the size of the tumor was about 17 cm. Surgical intervention should ideally be performed after 12 to 14 weeks of gestation. In our case, the operation was performed at 13 weeks of gestation. An exploratory laparotomy was performed as indicated in case of suspected malignancy since the risk of spillage of dermoid content increases with laparoscopic management [10]. Analogous to any ovarian cancer, optimal cytoreduction is associated with significantly improved survival. Tseng et al. [11] reported a disease-free survival for 2 years in 100% of patients with the International Federation of Gynecology and Obstetrics (FIGO) stage I disease. While para-aortic and pelvic lymph node dissection is controversial since the tumor spreads by direct extension and/or peritoneal seeding, metastatic lymph nodes have been described in the literature [11]. In our case, lymph node dissection was not performed as the report from frozen section revealed MCT. Due to the rarity of this disease, there is no sufficient data supporting the safety of fertility-sparing surgery even for early stage disease. Thus, complete surgical staging as performed in case of ovarian cancer (i.e., total hysterectomy, bilateral salpingo-oophorectomy, omentectomy and lymphonodectomy) is opted as the safest approach. There is no consensus regarding adjuvant treatment and effectiveness of chemotherapy or radiotherapy for ovarian SCC. Tseng et al. [11] reported a disease-free survival of 100% in four 1A patients without adjuvant treatment. Patients with IIB-IIIC disease were treated with cisplatinum-based chemotherapy with or without sequential radiotherapy. In our case, only a left salpingo-oophorectomy and right ovarian cystectomy was performed without adjuvant treatment. Dos Santos et al. [12] proposed whole-pelvis radiation and platinum-based chemotherapy for patients with stage I-II disease since SCC is a radiosensitive tumor. The prognosis of this tumor heavily depends on the FIGO stage. Other prognostic factors include tumor grade, growth pattern, capsular rupture and vascular invasion [13].
In premenopausal women, focal FDG uptake is often identified in the normal ovaries and could cause misinterpretation of the FDG PET-CT scan [14]. Kim et al. [15] retrospectively reviewed FDG PET-CT findings in 449 women patients with breast cancer, cervical cancer and endometrial cancer or as part of a regular checkup. Their results revealed focal ovarian uptake in 19 cases, 15 of which did not have FDG uptake on short-term follow-up PET-CT. For some cases, the disappearance of the uptake on a follow-up scan following subsequent menstruation could confirm benign uptake of the ovaries in the earlier scan [15]. In our case, every focal uptake of the ovary disappeared on a follow-up scan.
In summary, our patient developed SCC from an MCT during pregnancy, was treated conservatively without adjuvant chemotherapy and exhibited no evidence of disease for more than 60 months following the initial surgery.
Figures and Tables
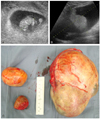 | Fig. 1(A) Sonography shows intrauterine pregnancy at 8+5 weeks of gestation. (B) Sonography shows about 18 cm solid and cystic left ovarian mass. (C) The specimen obtained shows huge cystic and solid left ovary with smooth surface and two small right ovarian cysts. |
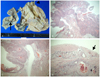 | Fig. 2(A) The ovarian cyst filled with sebaceous material shows a protuberance covered with hair tuffs (arrowhead). Cut surface of the protuberance discloses a cauliflower-like solid mass (arrow) protruding into the cyst. (B) A typical squamous cell carcinoma arises in the epidermal component (H&E, ×12.5). (C) Transition from benign epidermal tissue to squamous cell carcinoma with invasive growth is evident (H&E, ×40). (D) Mature glial tissue is partly invaded by squamous cell carcinoma (arrow) (H&E, ×200). |
References
1. Giuntoli RL 2nd, Vang RS, Bristow RE. Evaluation and management of adnexal masses during pregnancy. Clin Obstet Gynecol. 2006. 49:492–505.
2. Stamp GW, McConnell EM. Malignancy arising in cystic ovarian teratomas. A report of 24 cases. Br J Obstet Gynaecol. 1983. 90:671–675.
3. Curling OM, Potsides PN, Hudson CN. Malignant change in benign cystic teratoma of the ovary. Br J Obstet Gynaecol. 1979. 86:399–402.
4. Ji HY, Kim TJ, Kim MJ, Lee EJ, Lee YY, Kim CJ, et al. A study for diagnosis of squamous cell carcinoma arising from mature cystic teratoma. Korean J Obstet Gynecol. 2009. 52:1258–1264.
5. Ulker V, Numanoglu C, Akbayir O, Akyol A, Tuncel A, Akca A, et al. Malignant transformation arising from mature cystic teratoma of the ovary: a report of six cases. J Obstet Gynaecol Res. 2012. 38:849–853.
6. Oehler MK, Wain GV, Brand A. Gynaecological malignancies in pregnancy: a review. Aust N Z J Obstet Gynaecol. 2003. 43:414–420.
7. Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006. 101:315–321.
8. Mori Y, Nishii H, Takabe K, Shinozaki H, Matsumoto N, Suzuki K, et al. Preoperative diagnosis of malignant transformation arising from mature cystic teratoma of the ovary. Gynecol Oncol. 2003. 90:338–341.
9. Kido A, Togashi K, Konishi I, Kataoka ML, Koyama T, Ueda H, et al. Dermoid cysts of the ovary with malignant transformation: MR appearance. AJR Am J Roentgenol. 1999. 172:445–449.
10. Mayer C, Miller DM, Ehlen TG. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol Oncol. 2002. 84:180–183.
11. Tseng CJ, Chou HH, Huang KG, Chang TC, Liang CC, Lai CH, et al. Squamous cell carcinoma arising in mature cystic teratoma of the ovary. Gynecol Oncol. 1996. 63:364–370.
12. Dos Santos L, Mok E, Iasonos A, Park K, Soslow RA, Aghajanian C, et al. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: a case series and review of the literature. Gynecol Oncol. 2007. 105:321–324.
13. Rim SY, Kim SM, Choi HS. Malignant transformation of ovarian mature cystic teratoma. Int J Gynecol Cancer. 2006. 16:140–144.
14. Hansen TN, Brockbank KG. Increased platelet aggregation due to chilling to 20 degrees C is not related to increased sensitivity to agonists. Transfusion. 1997. 37:696–702.
15. Kim SK, Kang KW, Roh JW, Sim JS, Lee ES, Park SY. Incidental ovarian 18F-FDG accumulation on PET: correlation with the menstrual cycle. Eur J Nucl Med Mol Imaging. 2005. 32:757–763.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download