Abstract
Background
We determined the prevalence of sarcopenia according to fracture site and evaluated the associated risk factors in female patients with osteoporotic fractures.
Methods
A total of 108 patients aged 50 years or older with an osteoporotic fracture (hip, spine, or wrist) were enrolled in this retrospective observational study. A diagnosis of sarcopenia was confirmed using whole-body densitometry for skeletal muscle mass measurement. Logistic regression analysis was used to analyze the risk factors for sarcopenia.
Results
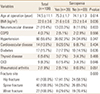
Of 108 female patients treated for osteoporotic fractures between January 2016 and June 2017, sarcopenia was diagnosed in 39 (36.1%). Of these, 41.5% (17/41) had hip fractures, 35% (14/40) had spine fractures, and 29.6% (8/27) had distal radius fractures. Body mass index (BMI; P=0.036) and prevalence of chronic kidney disease (CKD; P=0.046) and rheumatoid arthritis (P=0.051) were significantly different between the groups. In multivariable analysis, BMI (odds ratio [OR], 0.76; 95% confidence interval [CI], 0.55–1.05, P=0.098) and CKD (OR 2.51; 95% CI, 0.38–16.2; P=0.233) were associated with an increased risk of sarcopenia; however, this was not statistically significant.
Sarcopenia, or the progressive loss of muscle mass and function, has enjoyed increasing attention since it was first included in the International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM).[1] It is presently well known that sarcopenia is associated with several metabolic diseases, such as diabetes, rheumatoid arthritis (RA), chronic renal failure, congestive heart failure, and chronic obstructive pulmonary disease.[2345] Several studies have demonstrated that the presence of sarcopenia in these patients predicts poor outcomes.[67]
Osteoporosis is also an important risk factor for sarcopenia, with sarcopenia and osteoporosis sharing common risk factors and biological pathways.[8] In this way, it has been reported that there is a higher prevalence of sarcopenia in patients with hip fractures compared with the normal population.[9] The combined effect of sarcopenia and osteoporosis represents a serious problem in the elderly, partially due to their propensity for falls and develop fragility fractures. Recently, one study has demonstrated that the rate of mortality was increased by 1.8-fold in men with osteosarcopenia and was significantly higher than in men with either sarcopenia or osteoporosis alone.[10]
Most studies have assessed the prevalence or effect of sarcopenia in osteoporotic fracture patients, but they have not identified related risk factors. Moreover, most have assessed the incidence of osteoporotic fractures in the hip, vertebrae, and distal radius; however, the prevalence of sarcopenia in and across these fracture sites have not been evaluated.
The purpose of the present study was to determine the incidence of sarcopenia in three major osteoporotic fracture patients and determine the risk factor of sarcopenia in these patients.
Between January 2016 and June 2017, we evaluated 249 patients over 50 years of age who were diagnosed with hip (femoral neck or intertrochanteric), spine (vertebrae) or wrist (distal radius fractures) and who underwent surgery or conservative treatment. Osteoporotic fractures were defined as fractures resulting from fall injuries and having T-scores ≤−2.5 at either the femoral neck or spine.[11] Among these patients, 34 were excluded since dual energy X-ray absorptiometry (DXA) was not performed or they experienced dementia or delirium. Furthermore, we excluded patients who had T-scores >−2.5 (n=86); experienced a high energy injury, such as a traffic accident (n=18); or developed a pathologic fracture (n=3). A total of 108 patients with osteoporotic fracture (41 hip fractures, 40 spine fractures, 27 distal radius fractures) were ultimately included in this retrospective case control study, which was conducted under Institutional Review Board approval (CHAMC 2017-11-003).
Bone mineral density (BMD) as well as fat and lean body mass were assessed with a single DXA in one hospital following the manufacturer's instructions (GE Lunar, Madison, WI, USA). BMD of L1 to L4, the femoral neck, and the total femur (excluding the ward triad) were measured for BMD. Determination of bone mineral score were based on previously published protocol. In determining the spine bone density score, we followed the International Society for Cinical Densitometry vertebrae measurement tool.
The upper and lower appendicular lean mass (ALM) were assessed for skeletal muscle mass. Furthermore, we used appendicular skeletal mass (ASM) as an index of relative muscle mass and calculated it as the sum of the upper and lower ALM divided by the height squared (ALM/height2). Rather than using the cut-off values for sarcopenia for men (<7.26 kg/m2) and women (<5.45 kg/m2) that have been used Western countries, we employed the adjusted criteria for the Asian population (male, <7.00 kg/m2; female, <5.40 kg/m2).[12]
Two orthopedic surgeons reviewed medical records to identify the medical history, current occupation, activities of daily life, medication history, previous falls, and incidence of fractures of each patient. The age; sex; body mass index (BMI); and incidence of diabetes, hypertension, chronic obstructive pulmonary disease, cardiovascular disease (angina, myocardial infarction), neuromuscular disease (Parkinson's disease), thyroid disease (hyper or hypothyroidism), site of fracture (hip and spine and distal radius), chronic kidney disease (CKD), and RA, were assessed to determine the relationship between these variables and between the two groups (sarcopenia or not).
Student's t-test or the Mann-Whitney U-test was used for normally and non-normally distributed continuous data, respectively. The χ2 test or Fisher's exact test was used to analyze normally and non-normally distributed categorical data. For all tests, a two-sided P-value of 0.05 was considered significant. To determine the risk factors of sarcopenia, multivariable regression analysis was performed. Statistical analyses were conducted with SPSS for Windows statistical package, version 16.0 (SPSS Inc., Chicago, IL, USA). The design and protocol of this study were approved by the Institutional Review Board of each hospital, which waived informed consent.
Of the 108 patients with osteoporotic fractures the prevalence of sarcopenia was 35.8% (39/109), including 41.5% (17/41) in the hip, 35.0% (14/40) in the vertebral fracture, and 29.6% (8/27) in the distal radius. BMI (P=0.036), CKD (P=0.046), RA (P=0.051) were significantly different between the two groups. The demographic data of the patients are shown in Table 1.
In the multivariable analysis, BMI (odds ratio [OR], 0.76; 95% confidence interval [CI], 0.55–1.05, P=0.098) and CKD (OR, 2.51; 95% CI, 0.38–16.2, P=0.233) were associated with an increased risk of sarcopenia; however, this was not statistically significant. The OR for RA could not be calculated since there was insufficient data in the control group.
The aim of the present study was to investigate the prevalence of sarcopenia in elderly female patients at various fracture locations and to assess the associations with other risk factors. Our results have shown that sarcopenia was significantly more prevalent in the order of hip fracture, vertebral fracture, and distal radius fracture. BMI and the incidence of both CKD and RA were significantly different between the two groups. After adjusting, logistic regression analyses revealed that lower either BMI or CKD were associated with an increased risk of sarcopenia; however, this was not significantly different.
Sarcopenia in patients with osteoporotic fractures has been documented regularly.[13] In this study, we found sarcopenia prevalence of 35.8% (39/109) in overall osteoporotic fractures, which is similar to previous studies that have reported an incidence ranging from 27.9% to 37.0%. [1415] Hong et al.[16] studied the prevalence of sarcopenia in elderly Chinese women and observed that 41.9% of women experienced a hip fracture, 33.9% experienced a vertebral fracture, and 24.5% experienced a distal radius fracture. This finding also very consistent with our findings, the difference in these prevalence rates may be related to age or BMI. Because osteoporosis is importantly related with sarcopenia in elderly populations, in whom the aging process impacts both bone and muscle.[8]
Our study showed that RA and CKD are possible related risk factors. Importantly, these chronic inflammatory diseases can not only result in joint and bone destruction, but also can result in a reduction in the strength and mass of the skeletal muscles. Also, chronic inflammation accompanying these diseases, a decrease in physical activity, chronic pain, and an increase in energy expenditure during rest can result in a decrease in lean body mass over the course of RA or CKD.[17] This ultimately worsens movement disability, contributes to a faster deterioration in the quality of life, and is likely to shorten longevity.[2] One study documented that, in women with RA and who are of a normal body weight, the adjusted OR of a loss of lean body mass was more than three times greater (OR, 3.41; P<0.05) than in the control group.[18] Meanwhile, kidney disease can cause muscle wasting via accelerated muscle protein breakdown, which is possibly mediated by impaired mitochondrial respiratory function or reduced muscle mitochondrial mass.[19]
This study has several limitations. First, this was a retrospective single-center study, and selection bias may have been introduced when we chose the hip fracture patient subjects. Second, function and gait speed were not evaluated in this study. Third, all included patients were female, thereby limiting the generalization of our results to men.
In conclusion, this is the first study that evaluated the risk factors associated with sarcopenia in patients with osteoporotic fractures. The prevalence of sarcopenia according to fracture site was 41.5% (hip), 35.0% (spine), and 29.6% (wrist), with low BMI, RA, CKD being possible risk factors. A long-term, observational study with a larger population is needed to validate our results.
Figures and Tables
References
1. Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10-CM) code. J Am Med Dir Assoc. 2016; 17:675–677.

2. Androga L, Sharma D, Amodu A, et al. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int Rep. 2017; 2:201–211.

3. Dudgeon D, Baracos VE. Physiological and functional failure in chronic obstructive pulmonary disease, congestive heart failure and cancer: a debilitating intersection of sarcopenia, cachexia and breathlessness. Curr Opin Support Palliat Care. 2016; 10:236–241.

4. Tsuchida K, Fujihara Y, Hiroki J, et al. Significance of sarcopenia evaluation in acute decompensated heart failure. Int Heart J. 2018; 59:143–148.

5. Okazaki R, Watanabe R, Inoue D. Osteoporosis associated with chronic obstructive pulmonary disease. J Bone Metab. 2016; 23:111–120.

6. Liccini A, Malmstrom TK. Frailty and sarcopenia as predictors of adverse health outcomes in persons with diabetes mellitus. J Am Med Dir Assoc. 2016; 17:846–851.

7. Cebron Lipovec N, Schols AM, van den Borst B, et al. Sarcopenia in advanced COPD affects cardiometabolic risk reduction by short-term high-intensity pulmonary rehabilitation. J Am Med Dir Assoc. 2016; 17:814–820.

8. Edwards MH, Dennison EM, Aihie Sayer A, et al. Osteoporosis and sarcopenia in older age. Bone. 2015; 80:126–130.

9. Yoo JI, Ha YC, Kwon HB, et al. High prevalence of sarcopenia in Korean patients after hip fracture: a case-control study. J Korean Med Sci. 2016; 31:1479–1484.

10. Newton DH, Kim C, Lee N, et al. Sarcopenia predicts poor long-term survival in patients undergoing endovascular aortic aneurysm repair. J Vasc Surg. 2018; 67:453–459.

11. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004; 34:195–202.

12. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014; 15:95–101.

13. Park HM, Ha YC, Yoo JI, et al. Prevalence of sarcopenia adjusted body mass index in the Korean woman based on the Korean national health and nutritional examination surveys. J Bone Metab. 2016; 23:243–247.

14. Drey M, Sieber CC, Bertsch T, et al. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016; 28:895–899.

15. Huo YR, Suriyaarachchi P, Gomez F, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc. 2015; 16:290–295.

16. Hong W, Cheng Q, Zhu X, et al. Prevalence of sarcopenia and its relationship with sites of fragility fractures in elderly Chinese men and women. PLoS One. 2015; 10:e0138102.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download