Abstract
Background
Breast cancer is one of the most common cancers affecting women and has a high incidence of bone metastasis, causing osteolytic lesions. The elevated expression of receptor activator of nuclear factor-κB ligand (RANKL) in cancer activates osteoclasts, leading to bone destruction. We previously reported that α-tocopheryl succinate (αTP-suc) inhibited interleukin-1-induced RANKL expression in osteoblasts. Here, we examined the effect of αTP-suc on osteolytic bone metastasis in breast cancer.
Methods
To examine the effect of αTP-suc on the metastatic capacity of breast cancer, MDA-MB-231-FL cells were injected into the left cardiac ventricle of BALB/c nude mice along with intraperitoneal injection of αTP-suc. The mice were then analyzed by bioluminescence imaging. To investigate the effect of αTP-suc on osteolysis, 4T1 cells were directly injected into the femur of BALB/c mice along with intraperitoneal injection of αTP-suc. Microcomputed tomography analysis and histomorphometric analysis of the femora were performed.
Results
αTP-suc inhibited cell migration and cell growth of 4T1 cells. In line with these results, bone metastasis of MDA-MB-231-FL cells was reduced in mice injected with αTP-suc. In addition, αTP-suc decreased osteoclastogenesis by inhibiting 4T1-induced RANKL expression in osteoblasts. Consistent with these results, 4T1-induced bone destruction was ameliorated by αTP-suc, with in vivo analysis showing reduced tumor burden and osteoclast numbers.
Cancer is generally known to be incurable when the disease metastasizes into bone. Bone metastasis causes devastating clinical features, including excessive bone pain, pathological bone fractures, hypercalcemia, and spinal cord compression.[1] Among the metastatic cancers, bone metastasis most commonly occurs in breast and prostate cancer, which account for 65% to 75% of patients with bone metastasis. Cancers of the lung, colon, stomach, bladder, uterus, rectum, thyroid, and kidney cause up to 30% of bone metastasis.[2]
Breast cancer is one of the most common cancers in women and has a high incidence of bone metastasis. The most common outcome of bone metastatic breast cancer is osteolytic lesions due to increased osteoclast differentiation and activity.[34] Osteolytic lesions develop as a result of an interaction between the cancer and the bone marrow niche. Previous studies have shown that osteoblasts in the bone lining expressing C-X-C motif chemokine ligand 12 supply the metastatic niche to cancer cells expressing the C-X-C motif receptor 4.[5] Bone metastatic breast cancer migrates into the osteoblast-rich area and then interacts with cells in the bone marrow, including osteoblasts, fibroblasts, and T cells, through the secretion of various inflammatory factors, such as prostaglandin E2 (PGE2), interleukin 1 (IL-1), and IL-6. This inflammatory environment triggers cells including fibroblasts, T cells, and osteoblasts to increase the expression of receptor activator of nuclear factor (NF)-κB ligand (RANKL), a crucial cytokine for osteoclast differentiation. Elevated RANKL expression promotes osteoclast differentiation, leading to osteolysis. Osteolysis releases growth factors that have accumulated in the bone matrix, such as transforming growth factor-β (TGF-β), insulin-like growth factor (IGF), and platelet-derived growth factor (PDGF). The growth factors released from the bone matrix in turn stimulate more cancer growth and migration, leading to accelerated bone destruction, forming a vicious cycle.[46]
Vitamin E consists of a group of eight structurally related compounds, four tocopherols (α, β, γ, and δ) and four tocotrienols (α, β, γ, and δ). Accumulating evidence suggests that the various vitamin E families have anti-cancer activity. The vitamin E family inhibits cell growth and cell migration and induces apoptosis of various cancer cells, such as colon cancer, lung cancer, prostate cancer, and breast cancer.[7] For example, Husain et al.[8] showed that γ- and δ-tocotrienol inhibit cell growth and cell survival of pancreatic cancers by inhibiting NF-κB, and Gysin et al. [9] showed that γ-tocopherol inhibits the cell cycle progression of osteosarcoma cells by down-regulation of the cyclin family. Moreover, various human clinical studies have shown that the vitamin E family is effective for cancer therapy.[710]
For bone metastatic osteolysis, elevated osteoclast activity can be a therapeutic target to prevent bone destruction and tumor growth by this vicious cycle. We previously reported that α-tocopheryl succinate (αTP-suc), an esterified compound of α-tocopherol (αTP), inhibits IL-1-induced RANKL expression in osteoblasts and thereby suppresses osteoclast differentiation.[11] Also, studies have shown that αTP-suc inhibits cell invasion and proliferation of cancers in vitro.[12] However, the effects of αTP-suc on the metastatic capacity of cancers in vivo and the ability of αTP-suc to prevent bone destruction by bone metastasis have not yet been elucidated.
In the present study, we investigated whether αTP-suc affects cancer migration in vivo. We also examined whether αTP-suc inhibits cancer-induced RANKL expression from osteoblasts and whether αTP-suc prevents osteolytic bone metastasis and bone destruction.
Macrophage colony-stimulating factor (M-CSF) and RANKL were purchased from PeproTech (Rocky Hill, NJ, USA). αTP-suc was purchased from Sigma-Aldrich (St. Louis, MO, USA). The 4T1 mouse breast cancer cell line and the MDA-MB-231 human breast cancer cell line were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM). Primary bone marrow cells from 7-week-old BALB/c female mice and primary osteoblasts from newborn BALB/c calvariae were prepared as described previously [13] and cultured in α-minimal essential medium (α-MEM). Cell culture media and serum were purchased from Invitrogen (Carlsbad, CA, USA) and complete medium was supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT, USA) and 1% penicillin-streptomycin (Invitrogen). MDA-MB-231-FL cells were generated from MDA-MB-231 cells by stable transfection of the firefly luciferase gene as described previously.[14]
Six-week-old female BALB/c and BALB/c nude mice were purchased from Charles River Korea. All animal procedures were reviewed and approved by the animal care committee of the Institute of Laboratory Animal Resources of Seoul National University.
Starting 1 day before cancer injection, αTP-suc (0.1 mg/head) or dimethyl sulfoxide (DMSO) was injected intraperitoneally every 2 days for each model. For intracardiac injections (n=5), BALB/c nude mice were anesthetized and injected with 100 mL phosphate buffered saline (PBS) or 2×105 MDA-MB-231-FL cells in 100 mL PBS into the left cardiac ventricle, a route of administration that allows for bone metastasis rather than lung infiltration of injected cells. After 7 days of injection, mice were subjected to bioluminescence analysis. For intrafemoral injections (n=5), 5 mL PBS or 1×104 4T1 cells in 5 µL PBS was injected into the femoral marrow space of left femur through the femoral condyle using a 28-gauge Hamilton syringe as previous described.[15] After 7 days of injection, mice were sacrificed and femora were collected.
The mice injected with MDA-MB-231-FL cells were intraperitoneally injected with 150 mg/kg D-luciferin (Xenogen, Hopkinton, MA, USA) in PBS 12 min before bioluminescence imaging. Imaging was conducted by using a charge-coupled device camera (IVIS 100, Xenogen; exposure time of 3 min, binning of 8, field of view of 15 cm, f/stop of 1, and no filter). Mice were anesthetized with isoflurane (2% vaporized in O2). For analysis, total photon flux (photons per second) was measured from the whole body, and a fixed region of interest in the hind limbs and the mandible/maxilla by using Living Image software (Xenogen). Bioluminescent signals within the region were normalized to the background luminescence taken over the same region from animals not injected with D-luciferin.
Three-dimensional (3D) images of femora were reconstructed by microcomputed tomography (micro-CT) scanning (SMX-90CT system; 90 kVp, 109 mA, and 180-ms integration time; Shimadzu, Kyoto, Japan). Trabecular bone parameters were calculated by using TRI 3D-BON (RATOC System Engineering Co., Tokyo, Japan). All micro-CT image acquisitions and analyses were carried out by an individual blinded to the composition of the experimental groups.
Decalcified femoral bones were embedded in paraffin blocks. Histological sections (6 mm) were prepared and stained with hematoxylin and eosin (H & E) or with tartrate-resistant acid phosphatase (TRAP) to detect osteoclasts as described previously.[14] Histomorphometric analyses were conducted with the Osteomeasure analysis system (Osteo-Metrics, Decatur, GA, USA).
The 4T1 cells were harvested by using a cell dissociation solution (Sigma-Aldrich) and then resuspended in serum-free DMEM. A total of 1×105 4T1 cells in 100 mL of serum-free medium were seeded in the upper chamber of transwell chambers (8-mm pore membranes; Corning) with the indicated reagents. The lower chamber was filled with 900 mL of 10% FBS-containing medium. After 12 hr, cells on the upper surface of the filter were wiped off with cotton swabs. Cells on the lower surface of the filter were fixed with 3.7% formaldehyde and methanol and stained with H & E. Stained cells were counted under a microscope.
Cell viability and proliferation were determined with the EZ-Cytox Cell Viability Assay Kit (Daeil Lab, Seoul, Korea) based on the cleavage of the tetrazolium salt to water-soluble formazan by succinate-tetrazolium reductase. Briefly, 2×103 4T1 cells were seeded on 96-well plates and cultured for 24 hr. Then, the culture medium was exchanged for media containing different concentrations of αTP-suc (0–10 µM). After 2 days of culture, cells were incubated with 20 µL of Ez-CyTox solution for 2 hr in a 37℃ incubator. Absorbance was measured at 450 nm.
Primary bone marrow cells (1×106 cells/well) and primary osteoblasts (1×105/well) were co-cultured with 4T1 cells (5×102 cells/ well) in 12-well (1.5 mL/well) tissue culture plates for 8 days in α-MEM complete medium. The cultures were replenished with fresh medium containing the same supplements every 2 days. At the end of the culture period, cells were fixed in 10% formalin for 10 min, permeabilized with 0.1% Triton X-100, and then stained for TRAP activity with the leukocyte acid phosphatase assay kit (Sigma-Aldrich).
The 4T1 cells were cultured in DMEM complete medium on 10-cm tissue culture dishes until optimal confluence (70%). Then the cells were cultured in fresh DMEM complete medium. CM was harvested after 24 hr of incubation, centrifuged at 2,000 rpm for 5 min, and stored at −80℃.
Primary osteoblasts (4×105/well) were cultured in 6-well tissue culture plates with or without 4T1-CM (30%) in the presence of DMSO or αTP-suc (10 µM). After 24 hr of culture, cells were harvested for real-time PCR analysis and the culture medium was collected for enzyme-linked immunosorbent assay (ELISA). Total RNA was prepared by using the RNeasy mini kit (Qiagen, Valencia, CA, USA), and cDNA was synthesized from 2 mg of total RNA by use of reverse transcriptase (Superscript II Preamplification System; Invitrogen). Real-time PCR was performed as described previously.[13] The primers were as follows: for mouse RANKL, (sense) TGGAAGGCTCATGGTTGGAT and (antisense) CATTGATGGTGAGGTGTGCA; for mouse osteoprotegerin (OPG), (sense) TGGAACCCCA-GAGCGAAACA and (antisense) GCAGGAGGCCAAATGTGCTG; for mouse b-actin, (sense) ATGTGGATCAGCAAG-CAGGA and (antisense) AAGGGTGTAAAACGCAGCTC.
The protein levels of mouse RANKL and mouse OPG in samples collected as indicated above were measured by using the corresponding ELISA kits (R & D Systems) according to the manufacturer's instructions.
Data are presented as the mean±standard deviation (SD; for in vitro data) or the mean±standard error of the mean (SEM; for in vivo data). Statistical analysis was performed by either unpaired, two-tailed Student's t-test or one-way ANOVA followed by Dunnett's test using Graph-Pad Prism 5.0 (GraphPad Software, San Diego, CA, USA). All P-values of less than 0.05 were considered to indicate statistical significance.
Studies have shown that αTP-suc inhibits the invasiveness and cell growth of 4T1 cells, a breast cancer cell line. [1216] Thus, we confirmed the effect of αTP-suc in our experimental condition. As shown in Figure 1A and B, serum-induced cell migration of 4T1 cells was significantly inhibited by αTP-suc in a dose-dependent manner. Cell viability was also decreased by αTP-suc in a dose-dependent manner (Fig. 1C). The cell migration of 4T1 cells was inhibited by αTP-suc at 1 µM by approximately 50% in 12 hr. However, αTP-suc inhibited 4T1 cell growth by about 50% at 10 µM for 48 hr suggesting that inhibition of cell migration by αTP-suc was not due to inhibited cell growth by αTP-suc. These findings suggested that αTP-suc effectively inhibited cell mobility and proliferation in our laboratory conditions as reported previously.
Next, to determine whether αTP-suc is effective in vivo as demonstrated by the in vitro experiments, we used a cardiac injection mouse model. MDA-MB-231 human breast cancer cells expressing firefly luciferase (MDA-MB-231-FL) were injected into the left ventricle, and starting 1 day before cancer injection, αTP-suc was injected intraperitoneally every 2 days (Fig. 2A). After 7 days of cancer injection, bioluminescence imaging analysis was performed to determine the metastatic status of the cancer cells. In the bones, MDA-MB-231-FL cells mainly metastasized into hind limbs and mandible/maxilla (Fig. 2B). However, the mice injected with αTP-suc showed significantly less tumor burden (Fig. 2B). Tumor-induced total flux of the whole body, hind limbs, and mandible/maxilla were also decreased in αTP-suc-injected mice (Fig. 2C). These results showed that αTP-suc effectively inhibited cancer metastasis in vivo.
We previously reported that αTP-suc inhibits IL-1-promoted RANKL expression in osteoblasts.[16] Breast cancer cells secrete various factors, including IL-1, IL-6, and PGE2, that increase RANKL expression in osteoblasts, leading to enhanced osteoclastogenesis. Therefore, we investigated whether αTP-suc inhibited cancer-induced osteoclast differentiation in a triple co-culture of mouse primary osteoblasts, mouse primary bone marrow cells, and 4T1 cells. As shown in Figure 3A and B, 4T1-induced osteoclast differentiation was greatly reduced by αTP-suc (Fig. 3A, B). Next, we performed real-time PCR analysis and enzyme immunoassay to investigate the effect of αTP-suc on RANKL expression in osteoblasts induced by 4T1-CM. Osteoblasts treated with 4T1-CM showed elevated expression of RANKL, which was inhibited by αTP-suc in a dose-dependent manner (Fig. 3C). However, expression of OPG, a decoy receptor of RANKL, was not affected by αTP-suc (Fig. 3C). To confirm these results, we examined protein levels in the culture media. As shown in Figure 3D, the 4T1-CM-promoted expression of RANKL protein in the culture media of osteoblasts was also decreased by αTP-suc (Fig. 3D). These data suggested that αTP-suc inhibited the usual increase in RANKL expression in response to the secretion of factors from 4T1 cells in osteoblasts, leading to reduced osteoclastogenesis.
When cancer migrates into bones, communication between the bone marrow niche and the cancer cells forms a vicious cycle, accelerating bone destruction. Thus, to investigate whether αTP-suc affects bone destruction by this vicious cycle, 4T1 cells were directly injected into femora, and starting 1 day before cancer injection, αTP-suc was injected intraperitoneally every 2 days (Fig. 4A). Reconstructed images by micro-CT showed that the significant reduction in trabecular bones resulting from injection of 4T1 cells was greatly rescued in mice co-injected with αTP-suc (Fig. 4B). The trabecular bone parameters analyzed by micro-CT showed the same tendencies as the reconstructed images. The reductions in bone volume, trabecular thickness, and trabecular number induced by 4T1 cells were relieved in mice co-injected with αTP-suc (Fig. 4C). Histomorphometric analysis showed that tumor burden and the number of osteoclasts at the tumor-bone interface were significantly reduced in the αTP-suc-injected mice (Fig. 5A-D). These data demonstrated that αTP-suc suppressed the osteoclast formation and tumor growth induced by metastatic cancer, ameliorating the bone destruction.
Once bone metastasis of cancer occurs, it has historically been incurable, and patients with bone metastasis of breast cancer have a reported 5-year survival rate of only 20%.[1] Thus, it is urgent to develop therapeutic approaches to prevent and treat bone metastasis of breast cancer. In the present study, we found that αTP-suc (4 mg/kg every other day, delivered intraperitoneally) not only inhibited metastasis into bone but also decreased the tumor burden in bone and bone destruction by bone metastatic breast cancer cells.
Vitamins are organic compounds abundant in nature. Many studies have shown that various vitamins have anti-cancer effects. Bioactive vitamins including vitamins A, C, D, E, and K have anti-cancer potency such as inhibiting cell growth, differentiation, and migration and promoting apoptosis of cancer cells.[101718192021] The vitamin E family exhibits various functions including not only anti-cancer but also antioxidant, anti-inflammatory, and anti-thrombolytic activities.[212223] Among the vitamin E family, αTP is the most abundant in nature and in the human body and is expected to be useful as a supplement to prevent chronic diseases associated with oxidative stress.[24] In addition, αTP has the highest antioxidant capacity among the tocopherols in the following order: αTP >βTP >γTP >δTP.[25] αTP-suc is the most effective form of αTP for cancer therapy.[26] In vitro studies have shown the anti-cancer effect of αTP-suc. [1216] In addition, it was reported that cancer treatment is improved by using αTP-suc as an adjunct to radiation and chemotherapy.[26] In the present study, we demonstrated the in vivo effect of αTP-suc on cancer migration. As shown in Figure 2, metastasis of MDA-MB-231 cells into the whole body was greatly reduced in mice injected with αTP-suc (Fig. 2B, C). In addition, mice injected with αTP-suc showed minimal metastasis into the mandible/maxilla and limbs (Fig. 2B, C). Taken together, these results demonstrated that αTP-suc is also effective for preventing metastasis of breast cancer cells, especially metastasis into bone, in vivo. However, additional studies are required to compare the anti-cancer effect of the different αTP derivatives in vivo.
The mechanism of bone metastasis is complex and involves cooperative, reciprocal interactions among cancer cells, bone marrow cells, and the mineralized bone matrix. The excess of soluble and cellular components, the signaling network, and coordinated gene expression have been shown to contribute to the interplay among bone degradation, bone formation, and tumor growth. The interaction between the metastatic tumor and the bone marrow has been commonly referred as the “vicious cycle”.[27] This vicious cycle leads to two separated physiological phenomenon: osteolytic or osteoblastic bone metastasis, which depends on the type of cancer.[2728] Among the cancers, breast cancer undergoes osteolytic bone metastasis, which leads to overall bone loss.[4] The molecular mechanisms of bone destruction by metastatic breast cancer are well established. The migrated cancer cell initiates a vicious cycle by secreting inflammatory factors including parathyroid hormone-related protein, IL-1, IL-6, and PGE2. These inflammatory factors work on the osteoblasts, leading to increased expression of RANKL. The RANKL expressed from osteoblasts promotes osteoclast differentiation and activation, and the activated osteoclasts destroy bones. The destructed bone matrix releases growth factors that have accumulated in the bone such as TGF-β, IGF-1, and PDGF. These growth factors promote cell growth of metastatic tumors, promoting the release of more inflammatory factors.[4629] In addition, cancer-induced factors also stimulate RANKL expression in CD4+ T cells, contributing to bone destruction. [30] Moreover, RANKL+ regulatory T cells express more RANKL by breast cancer cells and then stimulate cancer metastasis.[31] The vicious cycle of bone metastasis also causes systemic inflammation, which promotes severe metastasis and low bone mineral density.[3233] Therefore, communication between cancer cells and bone marrow niches can be a therapeutic target for preventing osteolytic bone destruction. We previously reported that Trolox, a hydrophilic derivative of αTP, inhibits osteolytic bone metastasis by inhibiting cancer-induced RANKL expression from osteoblasts, but that αTP had no such effect.[1334] In addition, our previous report showed that αTP-suc inhibits IL-1-induced RANKL expression in osteoblasts and prevents osteoclast differentiation and bone resorption.[11] In the present study, we showed that αTP-suc also strongly inhibits cancer-induced RANKL expression in osteoblasts, leading to the inhibition of osteoclast differentiation (Fig. 3). These results indicate that αTP-suc can effectively inhibit cancer-induced RANKL expression, leading to the prevention of osteoclastogenesis.
Various animal models are established for the study of bone metastasis. The ideal model should be clinically relevant, recapitulate the human disease, and be reproducible. Thus, the best method is to inject cancer cells directly into the primary site and study the individual cells that have metastasized to the bone over time. However, the incidence of bone metastasis is very low and animals usually cannot survive until bone metastasis occurs. Therefore, a method of injecting cancer cells into blood vesicles, such as the left cardiac ventricle or the tail vein, is used to study bone metastasis. Of these methods, cardiac injection is considered a better method because the intravenous injection of cancer cells generally results in the accumulation of cancer cells in the lungs.[35] In the present study, we used intracardiac injection of MDA-MB-231-FL cells (Fig. 2). Metastasis of MDA-MB-231-FL cells into the whole body, including bones, was greatly reduced in αTP-suc-injected mice (Fig. 2). Because αTP-suc inhibited cell migration in the intracardiac injection model, we next used intrafemoral injection of 4T1 cells to study the effect of αTP-suc on the interaction between cancers and bone marrow cells after metastasis of cancer cells into bone (Fig. 4). As shown in Figure 4 and 5, the 4T1-induced bone destruction, tumor burden, and number of osteoclasts were greatly reduced by αTP-suc injection (Fig. 4, 5). These results indicated that αTP-suc was effective for inhibiting bone destruction and tumor growth.
In the present study, we showed that αTP-suc has dual effects on bone metastatic breast cancers: inhibition of cell growth and migration of cancer, and suppression of the vicious cycle by decreasing cancer-derived-factor-induced RANKL expression in osteoblasts. Therefore, the results of the present study indicate that αTP-suc can be efficiently utilized to prevent and treat osteolytic bone metastasis of breast cancer with dual effects.
Figures and Tables
 | Fig. 1Effect of α-tocopheryl succinate (αTP-suc) on the cell migration and cell viability of 4T1 cells. (A, B) Cell migration of serum-starved 4T1 cells in response to 10% fetal bovine serum in the presence of dimethyl sulfoxide (DMSO) or different concentrations of αTP-suc (1–10 µM) was assessed in transwell chambers for 12 hr. (A) Representative images of hematoxylin and eosin staining, ×100 magnification; and scale bar, 200 µm. (B) The number of migrated 4T1 cells per field (n=6 per each well). (C) The 4T1 cells were cultured with DMSO or different concentrations of αTP-suc (1–10 µM) for 2 days and then cell viability was measured. The results shown are representative of three independent experiments (n=3), and the values are expressed as mean±standard deviation. ***P<0.001 vs. vehicle treatment by unpaired Student's t-test. FBS, fetal bovine serum. |
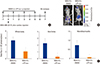 | Fig. 2Effect of α-tocopheryl succinate (αTP-suc) on metastasis by intracardiac injection of MDA-MB-231-FL (MDA-FL) cells. MDA-MB-231-FL cells or phosphate-buffered saline was injected into the left cardiac ventricle and metastasis of cancer was analyzed on day 7 after injection of cancer. Starting 1 day before cancer injection, αTP-suc (0.1 mg/head) or dimethyl sulfoxide (DMSO) was injected intraperitoneally every 2 days (n =5 each). (A) Scheme of the intracardiac injection mouse model. (B) Representative bioluminescence images. (C) Bioluminescence imaging analysis of tumor burden in the whole body. The values are expressed as mean±standard error of the mean. †P<0.05 vs. DMSO-injected mice with injection of MDA-MB-231-FL cells by one-way repeated-measures analysis of variance followed by Dunnett's test. i.p., intraperitoneally. |
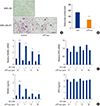 | Fig. 3Effect of α-tocopheryl succinate (αTP-suc) on osteoclast differentiation in triple co-culture and on 4T1 cell-conditioned medium (4T1-CM)-induced receptor activator of nuclear factor-κB ligand (RANKL) expression in osteoblasts (OBs). (A, B) Primary bone marrow cells and OBs were co-cultured with or without 4T1 cells in the presence of dimethyl sulfoxide (DMSO) or αTP-suc (10 µM) for 8 days. (A) Representative images of tartrate-resistant acid phosphatase staining, ×100 magnification; and scale bar, 200 µm. (B) The number of osteoclasts. ***P<0.001 vs. DMSO-treated control by unpaired Student's t-test. (C, D) OBs were cultured with or without 4T1-CM (30%) in the presence of DMSO or different concentrations of αTP-suc (1–10 µM) for 24 hr. (C) Levels of RANKL messenger RNA (mRNA) (left panel) and osteoprotegerin (OPG) mRNA (right panel) were measured by real-time polymerase chain reaction analysis. (D) Levels of RANKL protein (left panel) and OPG protein (right panel) in the culture media were measured by enzyme-linked immunosorbent assay. The results shown are representative of three independent experiments (n=3), and the values are expressed as mean±standard deviation. ***P<0.001 vs. untreated control and †††P<0.001; ††P<0.01; †P<0.05 vs. DMSO-treated cells in response to 4T1-CM by unpaired Student's t-test. BMC, bone marrow cell. |
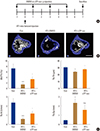 | Fig. 4Effect of α-tocopheryl succinate (αTP-suc) on the bone destruction by 4T1 cells in the mouse model of intrafemoral injection. The 4T1 cells were directly injected into the left femur. Starting 1 day before cancer injection, αTP-suc (0.1 mg/head) or dimethyl sulfoxide (DMSO) was injected intraperitoneally every 2 days (n=5 each). After 7 days of 4T1 injection, left femora were collected and analyzed by microcomputed tomography (micro-CT) scanning. (A) Scheme of the intrafemoral injection mouse model. (B) Representative reconstructed images by micro-CT (scale bar, 0.7 mm). (C) Quantification of trabecular bone parameters: bone volume per total volume (BV/TV, left upper panel), trabecular thickness (Tb.Th, right upper panel), trabecular number (Tb.N, left bottom panel), and trabecular separation (Tb.Sp, right bottom panel). The values are expressed as mean±standard error of the mean. ***P<0.001; *P<0.05 vs. phosphate-buffered saline-injected mice and †P<0.05 vs. DMSO-injected mice with injection of 4T1 cells by one-way repeated-measures analysis of variance followed by Dunnett's test. n.s., not significant; i.p., intraperitoneally. |
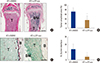 | Fig. 5Effect of α-tocopheryl succinate on tumor burden and osteoclast number by 4T1 cells. Histological analysis of bone metastasis from the experimental groups in Figure 4 (n=5). (A) Representative images of hematoxylin and eosin (H & E)-stained femur sections, ×100 magnification; and scale bar, 500 µm. (B) Histomorphometric quantification of tumor burden from H & E sections. (C) Representative images of tartrate-resistant acid phosphatase (TRAP)-stained femur sections, ×400 magnification; and scale bar, 200 µm. (D) Histomorphometric quantification of osteoclast numbers from TRAP-stained sections. †P<0.05 vs. dimethyl sulfoxide (DMSO)-injected mice with injection of 4T1 cells by one-way repeated-measures analysis of variance followed by Dunnett's test. T, tumor; BM, bone marrow; B, bone; αTP-suc, α-tocopheryl succinate. |
ACKNOWLEDGMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1A2B2002312) and the research funding from Korean Society for Bone and Mineral Research.
References
2. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001; 27:165–176.

3. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002; 2:584–593.

4. Chen YC, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res. 2010; 12:215.

5. Wang N, Docherty FE, Brown HK, et al. Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis: evidence from in vivo models. J Bone Miner Res. 2014; 29:2688–2696.

6. Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016; 16:373–386.

7. Ju J, Picinich SC, Yang Z, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010; 31:533–542.

8. Husain K, Francois RA, Yamauchi T, et al. Vitamin E delta-to-cotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Mol Cancer Ther. 2011; 10:2363–2372.

9. Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002; 16:1952–1954.

11. Kim HN, Lee JH, Jin WJ, et al. alpha-Tocopheryl succinate inhibits osteoclast formation by suppressing receptor activator of nuclear factor-kappaB ligand (RANKL) expression and bone resorption. J Bone Metab. 2012; 19:111–120.

12. Savitskaya MA, Onischenko GE. alpha-Tocopheryl succinate affects malignant cell viability, proliferation, and differentiation. Biochemistry (Mosc). 2016; 81:806–818.

13. Lee JH, Kim HN, Yang D, et al. Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling. J Biol Chem. 2009; 284:13725–13734.

14. Lee JH, Kim HN, Kim KO, et al. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res. 2012; 72:3175–3186.

15. Jin WJ, Kim B, Kim D, et al. NF-kappaB signaling regulates cell-autonomous regulation of CXCL10 in breast cancer 4T1 cells. Exp Mol Med. 2017; 49:e295.
16. Wang D, Chuang HC, Weng SC, et al. alpha-Tocopheryl succinate as a scaffold to develop potent inhibitors of breast cancer cell adhesion. J Med Chem. 2009; 52:5642–5648.

17. Davis-Yadley AH, Malafa MP. Vitamins in pancreatic cancer: a review of underlying mechanisms and future applications. Adv Nutr. 2015; 6:774–802.

18. Doldo E, Costanza G, Agostinelli S, et al. Vitamin A, cancer treatment and prevention: the new role of cellular retinol binding proteins. Biomed Res Int. 2015; 2015:624627.

19. Fritz H, Flower G, Weeks L, et al. Intravenous vitamin C and cancer: A systematic review. Integr Cancer Ther. 2014; 13:280–300.
20. Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006; 96:252–261.

21. Lamson DW, Plaza SM. The anticancer effects of vitamin K. Altern Med Rev. 2003; 8:303–318.
22. Frank J. Beyond vitamin E supplementation: an alternative strategy to improve vitamin E status. J Plant Physiol. 2005; 162:834–843.

23. Azzi A, Ricciarelli R, Zingg JM. Non-antioxidant molecular functions of alpha-tocopherol (vitamin E). FEBS Lett. 2002; 519:8–10.
24. Diplock AT. Safety of antioxidant vitamins and beta-carotene. Am J Clin Nutr. 1995; 62:1510s–1516s.

25. Müller L, Theile K, Böhm V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol Nutr Food Res. 2010; 54:731–742.

26. Prasad KN, Kumar B, Yan XD, et al. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. J Am Coll Nutr. 2003; 22:108–117.

27. Futakuchi M, Fukamachi K, Suzui M. Heterogeneity of tumor cells in the bone microenvironment: mechanisms and therapeutic targets for bone metastasis of prostate or breast cancer. Adv Drug Deliv Rev. 2016; 99:206–211.

28. Clezardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis. 2007; 24:599–608.

29. Hauschka PV, Chen TL, Mavrakos AE. Polypeptide growth factors in bone matrix. Ciba Found Symp. 1988; 136:207–225.

30. Monteiro AC, Leal AC, Gonçalves-Silva T, et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS One. 2013; 8:e68171.
31. Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011; 470:548–553.

32. Chechlinska M, Kowalewska M, Nowak R. Systemic inflammation as a confounding factor in cancer biomarker discovery and validation. Nat Rev Cancer. 2010; 10:2–3.

33. Okazaki R, Watanabe R, Inoue D. Osteoporosis associated with chronic obstructive pulmonary disease. J Bone Metab. 2016; 23:111–120.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download