Abstract
Background
The purpose of this prospective, open-label, observational study was to assess the fracture preventing effect of Maxmarvil® tablets (alendronate 5 mg + calcitriol 0.5 µg) in patients with osteoporosis and to evaluate the change in bone mineral density (BMD) at the minimum 1-year follow-up.
Methods
In this multicenter observational study, 691 patients with osteoporosis (aged 50 years or older) were treated with alendronate 5 mg + calcitriol 0.5 µg/day during their normal course of care. Patients were assessed at baseline and at 6 and 12 months. Baseline characteristics (including age, gender, concomitant disease, and baseline fractures) were evaluated.
Results
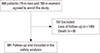
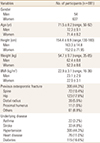
From among the 848 participants, 149 individuals were lost to follow-up at the time of the study and 8 people had died. The 691 participants (54 men and 637 women) finished the follow-up study and completed the questionnaire. The mean age of the participants was 71.5 years (range, 50–92 years; mean age, 72.3 years for men and 71.4 years for women). Osteoporotic fracture occurred in 19 patients (2.7%). BMD of the lumbar spine and hip was improved by 5% and 1.5% at the latest follow-up. At the latest follow-up, 24 patients (3.5%) complained of drug-related complications such as dyspepsia, constipation, and nausea.
Osteoporosis is an increasing concern for older adults as painful fragility fractures can significantly affect the overall health and quality of life.[1234] In South Korea, the lifetime risk of fracture at the age of 50 is estimated to be 59.6% for women and 23.5% for men.[5]
Anti-osteoporosis medication is important for preventing osteoporotic fracture and for decreasing mortality and morbidity.[678] Among them, bisphosphonates (BPs) are considered first-line therapy for the prevention and treatment of osteoporosis and they have demonstrated efficacy in the prevention of osteoporotic fractures at a variety of skeletal sites.[910] Clinical trials of each agent have demonstrated statistically significant reductions in the risk of nonvertebral fractures.[91011]
Since 2004, a combination drug containing alendronate 5 mg plus calcitriol 0.5 µg (Maxmarvil®; Yuyu Co., Seoul, Korea) has been introduced and are available.[12] This combination drug is quite effective in maintaining the bone mass in Asian osteoporotic postmenopausal women, even though the daily dosage of alendronate approved by the U.S. Food and Drug Administration is 10 mg.[12] However, there is only one study assessing the fracture preventing effects and bone mineral density (BMD) change after using this combination drug.[12] The purpose of this prospective, open-label, observational study was to assess the preventive effect on osteoporotic fracture using Maxmarvil® tablets (alendronate 5 mg + calcitriol 0.5 µg) in patients with osteoporosis and to evaluate the change in BMD at the minimum 1-year follow-up.
The design and protocol of this prospective, open-label, observational multicenter study were approved by the Institutional Review Board of each hospital. Patients who were diagnosed with osteoporosis from January 2011 to December 2014 were eligible for this study. All patients were evaluated for age, gender, body mass index (BMI), BMD, concomitant disease, and baseline fractures.
Inclusion criteria were age 50 years and over, diagnosis of osteoporosis (T-score≤-2.5) using central dual energy X-ray Absorptiometry (DXA), and no history of cancer or infection. Exclusion criteria were use of antiosteoporotic medication prior to 3 months, contraindication for using BP, renal stone, and refusal to participate. Enrollment and exclusion decisions were made by an investigator who did not otherwise participate in the study.
The study, for which the data were collected during four patient visits, was designed to evaluate the prevalence of osteoporotic fracture and BMD change. At baseline (visit 1), all patients signed an Institutional Review Board-approved informed consent document, were evaluated for inclusion in the study, were questioned about their medical history and demographics, and were prescribed Maxmarvil® tablets as daily medication by their physicians. At visit 2 (12 weeks) and visit 3 (6 months), data were collected. At visit 4 (endpoint; 54 weeks from baseline), additional data were collected and the BMD was evaluated after 12 months of treatment.
Lumbar spine or/ and hip BMD was measured using DXA at each hospital. The BMD of the lumbar spine (L2-L4) or/and total hip and femur neck was measured at the commencement of the study and after 12 months of treatment. A statistically significant increase in BMD was defined as a change exceeding the least significant change (LSC).
Patients were asked to report new fractures occurring after the baseline visit, and information on the fracture site was collected (spine, hip, wrist, proximal humerus). All information regarding the fracture was self-reported by the patient and confirmed by X-ray assessments.
The primary outcome variables were incidence of osteoporotic fractures including the spine, hip, wrist, and humerus, and change in BMD at the minimal one-year follow-up period. The secondary outcome was prevalence of adverse events during the follow-up period.
The chi-square test or Fisher exact test used to analyze descriptive variables and the t-test was used to analyze numeric variables, as appropriate. Two-sided P-values<0.05 were considered statistically significant. Statistical analyses were performed using SPSS software version 19.0; IBM Corp., Armonk, NY, USA).
A total of 848 patients with osteoporosis (79 men and 769 women) were enrolled in this study. The mean age was 71.9 years (range, 50–97 years). Of these 848 patients, 691 patients (54 men and 637 women) finished the minimum 12-month follow-up (Fig. 1). The mean age of patients was 71.5 years (range, 50-92 years). Three hundred patients (306 cases) had a previous history of osteoporotic fracture including the spine (72 cases), hip (123 cases), distal radius (39 cases), proximal humerus (11 cases), and other sites (61 cases). Other concomitant comorbidities are described in Table 1.
During the follow-up period, 19 of the 691 patients (2 men and 17 women) (2.7%) developed an osteoporotic fracture (0.6% spinal fractures and 2.1% nonspinal fractures). Eighteen patients (6%) of 300 patients with previous osteoporotic fracture developed secondary osteoporotic fractures and 17 patients experienced fractures at the same anatomic site. Only one of the 391 patients without osteoporotic fracture (0.3%) developed a spinal fracture. Most of the osteoporotic fractures (17/19 patients, 89.5%) occurred at the 6-month follow-up (Table 2).
At the 12-month follow-up, in 57 patients, lumbar spine BMD could not be assessed, and in 47 patients, hip BMD could not be assessed, due to measurement error, instrumentation, or only one site (hip or lumbar spine) measurement. The pre-treatment average lumbar and hip BMD was 0.798±0.155 g/cm2 and 0.667±0.138 g/cm2, and the post-treatment average lumbar and hip BMD was 0.838±0.162 g/cm2 and 0.677±0.132 g/cm2, respectively (P<0.001 and P=0.006). The average improvements in lumbar and hip BMD were 5% and 1.5%, respectively (P<0.001) (Fig. 2A, B).
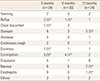
Forty adverse events in 34 patients were reported at the 3-month follow-up and 27 adverse events in 24 patients were reported at the 12-month follow-up. During the follow-up period, the incidence of adverse events decreased and the common type of adverse events changed at each visit. At the latest follow-up, dyspepsia was the most common adverse event and then upper gastric pain was reported (Table 3).
Although combination therapies of BP with vitamin D are popular and generalized, Maxmarvil® (5 mg alendronate with active vitamin D) is the only anti-osteoporosis medication combining alendronate and calcitriol. This study demonstrated that the incidence of osteoporotic fracture was 2.7% at the 12-month follow-up and lumbar spine and total hip BMD was increased by 5% and 1.5%, respectively.
Overall incidence of osteoporotic fracture in this study was 2.7% (0.6% spinal fractures, 2.1% non-spinal fractures). This finding is comparable with that in other studies. Watts et al. [13] retrospectively analysed 5,507 patients taking alendronate medications using administrative claims database, and they reported that 2.4% of non-spinal fractures occurred at the 12-month follow-up. Foster et al. [14] reported that the one-year incidence of spinal and non-spinal fractures was 0.3% and 2.2%, respectively after evaluating 172,939 patients taking alendronate using claims database. Modi et al. [15] evaluated the fracture rates among 26,852 women who were adherent to BP therapy for a minimum of 12 months using claims database, and they reported that the rate of fragility fractures was 3.9%. However, in this study, most of the osteoporotic fractures occurred at the 6-month follow-up.
At the 12-month follow-up, the mean improvement in lumbar and hip BMD was 5% and 1.5% in this study. This finding is similar to that in other previous studies. Rhee et al. [12] reported that an increase in BMD of 2.4%±0.5% after 24 weeks of 5 mg/day of alendronate with alfacalciferol. Iwamoto et al. [16] performed a prospective observational study in 105 postmenopausal women with 5 mg/alendronate, and they reported that the improvement in lumbar BMD was 8.2% at 12 months. In general, a dose of alendronate of 10 mg/day or 70 mg/week is most popular worldwide. Bonnick et al. [17] performed a randomized control study in 1053 women, and they reported that lumbar and hip BMD were improved by 5.2% and 3.0% at 2 years after using 70 mg/week alendronate. Tan et al. [18] reported that lumbar and hip BMD were improved by 5.8% and 3.6% at 1 year after using 70 mg/week alendronate. Although an improvement in BMD has been consistently reported in several studies, amount of improvement in BMD is slightly different. The most important reason for this discrepancy might be related to the patient's demographic characteristics and different amounts of alendronate.
The overall adverse event rate at 12 months was 3.5%. Suh et al. [19] evaluated the efficacy and safety in 568 patients treated with 5 mg/day alendronate along with 0.5 µg calcitriol, and they reported that the rate of adverse events was 5.6%. They reported abdominal pain and dyspepsia as the most common adverse events. These adverse events are commonly experienced by patients who are prescribed BPs, including those in this study.
In conclusion, this prospective observational study demonstrated that alendronate 5 mg + calcitriol 0.5 µg/day had a preventive effect on osteoporotic fracture and increased the lumbar BMD by 5% at the latest follow-up.
This study has several limitations. Firstly, this prospective observational study did not include a control group and it lacked randomization. Therefore, direct comparison of the effect using 5 mg/day alendronate with 0.5 µg calcitriol was not possible. However, we could observe a similar incidence of fracture and improvement in lumbar BMD. Secondly, there could be errors in the measured values because the DXA machines used in the patient group were different from those used in the control group. Finally, of the 848 patients, 157 patients were excluded due to several reasons. Although considering the dropout rate in similar studies using BP, a dropout rate of 17.7% might be acceptable, and this rate can possibly affect the outcomes.
Figures and Tables
Fig. 2
(A) Mean lumbar spine bone mineral density (BMD; g/cm2) and (B) mean hip BMD (g/cm2) after one year treatment with Maxmarvil®.

ACKNOWLEDGEMENTS
This research was supported by the Yuyu Pharma, Inc. with study approved as AJIRB-MED-CT1-10-335.
References
1. Ha YC, Kim TY, Lee A, et al. Current trends and future projections of hip fracture in South Korea using nationwide claims data. Osteoporos Int. 2016; 27:2603–2609.

2. Lee SR, Ha YC, Kang H, et al. Morbidity and mortality in Jeju residents over 50-years of age with hip fracture with mean 6-year follow-up: a prospective cohort study. J Korean Med Sci. 2013; 28:1089–1094.

3. Madureira MM, Ciconelli RM, Pereira RM. Quality of life measurements in patients with osteoporosis and fractures. Clinics (Sao Paulo). 2012; 67:1315–1320.

4. Abimanyi-Ochom J, Watts JJ, Borgström F, et al. Changes in quality of life associated with fragility fractures: Australian arm of the International Cost and Utility Related to Osteoporotic Fractures Study (AusICUROS). Osteoporos Int. 2015; 26:1781–1790.

5. Park C, Ha YC, Jang S, et al. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab. 2011; 29:744–751.

6. Peng J, Liu Y, Chen L, et al. Bisphosphonates can prevent recurrent hip fracture and reduce the mortality in osteoporotic patient with hip fracture: a meta-analysis. Pak J Med Sci. 2016; 32:499–504.

7. Center JR, Bliuc D, Nguyen ND, et al. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab. 2011; 96:1006–1014.

8. Cree MW, Juby AG, Carriere KC. Mortality and morbidity associated with osteoporosis drug treatment following hip fracture. Osteoporos Int. 2003; 14:722–727.

9. Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008; (1):CD001155.

10. Wells G, Cranney A, Peterson J, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008; (1):CD004523.

11. Zhou J, Ma X, Wang T, et al. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: a systematic review with network meta-analyses. Osteoporos Int. 2016; 27:3289–3300.

12. Rhee Y, Kang M, Min Y, et al. Effects of a combined alendronate and calcitriol agent (Maxmarvil) on bone metabolism in Korean postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Osteoporos Int. 2006; 17:1801–1807.

13. Watts NB, Worley K, Solis A, et al. Comparison of risedronate to alendronate and calcitonin for early reduction of nonvertebral fracture risk: results from a managed care administrative claims database. J Manag Care Pharm. 2004; 10:142–151.

14. Foster SA, Shi N, Curkendall S, et al. Fractures in women treated with raloxifene or alendronate: a retrospective database analysis. BMC Womens Health. 2013; 13:15.

15. Modi A, Tang J, Sen S, et al. Osteoporotic fracture rate among women with at least 1 year of adherence to osteoporosis treatment. Curr Med Res Opin. 2015; 31:767–777.

16. Iwamoto J, Takeda T, Sato Y, et al. Early changes in urinary cross-linked N-terminal telopeptides of type I collagen level correlate with 1-year response of lumbar bone mineral density to alendronate in postmenopausal Japanese women with osteoporosis. J Bone Miner Metab. 2005; 23:238–242.

17. Bonnick S, Saag KG, Kiel DP, et al. Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab. 2006; 91:2631–2637.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download