Abstract
Background
The use of osteoanabolic agents to facilitate fracture healing has been of heightened interest to the field of orthopaedic trauma. This study aimed to evaluate the evidence of teriparatide for fracture healing and functional recovery in osteoporotic patients.
Methods
We performed a literature search in PubMed, EMBASE, Web of Science, and the Cochrane Library using terms including “Fracture” [tiab] AND “Teriparatide [tiab] OR “PTH” [tiab].
Results
This systematic review included 6 randomized clinical trials, 4 well-controlled retrospective studies, and 1 retrospective post hoc subgroup analysis. Fracture location was 2 in pelvis, 3 in proximal femur, 1 in distal femur, 1 in shoulder, 2 in wrist and 2 in spine. The use of teriparatide yielded positive effects on radiographic bone healing in 6 studies, but was not associated with better radiographic outcome in 3. In terms of functional recovery, teriparatide injection was related with decrease in pain or shorter time to mobilization in 6 studies, but not related with pain numerical scale and mobility in 3.
Conclusions
Our findings suggest that teriparatide provide selective advantages to fracture healing or functional recovery in the management of osteoporotic fractures. A better understanding of the role of teriparatide on osteoporotic fractures requires greater evidences from large volume prospective trials.
Parathyroid hormone (PTH) is an important systemic regulator of calcium, phosphate, and active vitamin-D metabolites in human body. Amino-terminal PTH peptide fragments or analogs has been known to augment bone mass [1] and currently are being introduced into clinical practice as management for osteoporosis.[23]
Intermittent-injection recombinant human PTH (teriparatide) is the only anabolic agent approved by the U.S. Food and Drug Administration to increase bone mineral density (BMD) in osteoporotic patients. A large number of studies have reported the efficacy of teriparatide to prevent fractures in patients who have osteoporosis.[245678910] Findings from these studies have demonstrated significant increase of lumbar spine and femoral neck BMD and a lower risk of vertebral and non-vertebral fractures both in postmenopausal women and men.
Osteoporotic fractures generally occur from low-energy trauma and are common in elderly with comorbidities. Most patients with osteoporotic fractures sustained fracture site pain, impaired mobility with a large proportion failing to regain their prefracture functional status.[1112] Delayed or imperfect recovery after these fractures can be related with poor consequences. Multiple strategies, involving biophysical (electromagnetic field or ultrasound stimulation), local (autologous bone, calcium phosphates, and growth factors) and systemic (anti-Dickkopf-related protein I and anti-sclerostin antibodies) cell-based systemic therapies have been tried to overcome these problems.[3131415]
In recent decades, there has been a great interest in using an osteoanabolic agent to promote fracture healing. In animal models, teriparatide has shown the stimulatory effect on bone formation and acceleration of fracture healing.[161718] In some human study, it seemed to lower the risk of nonunion[19] and reduce healing time of atypical fractures.[2021] However, small studies to date have directly compared the fracture healing or functional recovery in controlled studies and their answer is still controversial.
This systematic review was conducted to retrieve and summarize clinical studies on the use of teriparatide for treatment of osteoporotic fractures and, thus determine the role of teriparatide in radiographic healing and functional recovery.
Osteoporotic (fragility) fracture is defined by the World Health Organization (WHO)[22] as “a fracture caused by injury that would be insufficient to fracture a normal bone”. Clinically a osteoporotic fracture may be defined as a fracture that occurs as a result of a minimal trauma, such as a fall from a standing height or less, or no identifiable trauma.[23] Reduced bone density is a major risk factor for osteoporotic fracture. However, lots of studies have demonstrated that the majorities of osteoporotic fracture occur in people who do not have osteoporosis.[2425] Fractures caused by low-level trauma equivalent to a fall from a standing height or less at sites presented above in adults aged over 50, should be first regarded as osteoporotic.[26] According to National Institute for Health and Care Excellence (NICE) guideline,[27] osteoporotic fractures occur most commonly in the spine, hip, and distal radius, but may also occur in the humerus, pelvis, ribs, and other bones. The WHO also considers proximal humerus fractures to be one of the major osteoporotic fractures.
This systematic review included clinical trials or retrospective case-controlled studies investigating the effect of teriparatide on fracture healing or functional status in osteoporotic fracture. Multiple comprehensive databases, including PubMed, EMBASE, Web of Science, and the Cochrane Library were searched for retrieving articles that were published before December, 2016. Search terms included “Fracture” [tiab] AND “Teriparatide [tiab] OR “PTH” [tiab]. Google Scholar was also used to screen relevant literatures. After initial research, relevant articles and their bibliographies were searched manually. Non-English language studies, standing-alone abstracts, meeting presentations, commentaries, and review articles were excluded. Articles not relevant to the use of teriparatide in fracture were also excluded. Then, the full texts of all retrieved articles were individually reviewed for inclusion. Studies were included in the systematic review if they (1) assessed variables on fracture healing of osteoporotic fracture; (2) reported variables on functional outcome after osteoporotic fracture in adults over age 50. Exclusion criteria included articles investigating studies without a control group, reporting on non-human subjects, and related with metabolic disorder except osteoporosis. This study was waived from institutional review board review because it did not involve human subjects.
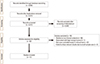
A total of 87 published articles investigating the use of teriparatide in fracture were identified (Fig. 1). Of these 87 studies, 46 which evaluated fracture healing on non-human subjects were excluded. One excluded because their fractures occurred from high energy trauma. Twenty case reports and 7 case series, which were considered to have substantial limitation to establish evidence, were also excluded. Finally, 11 studies met the inclusion criteria in this systematic review. The detailed information of these articles is summarized in Table 1.
Of these 11 articles, 4 were retrospective controlled series, 6 were assessed as being of high quality randomized clinical trials (RCTs), and 1 was retrospective post hoc subgroup analysis. Fracture location was 2 in pelvis, 3 in proximal femur, 1 in distal femur, 1 in shoulder, 2 in wrist and 2 in spine. Used regimen of the patient group was teriparatide in all studies except 1 (PTH 1-84) and that of the control group was placebo in 8 studies and risedronate in 3. Calcium plus vitamin D was supplemented in 8 studies.
Teriparatide was injected subcutaneously in a dosage of 20 µg once daily in all of studies except 1. Aspenberg et al. [28] tested the hypothesis that teriparatide 40 µg would shorten the time to cortical bridging in distal radial fractures, but this hypothesis was not supported. Treatment period of teriparatide ranged from 4 weeks to11 months. In 1 study evaluating pubic bone fractures, PTH 1-84 continued for 24 months after the fractures had healed. Fracture healing was evaluated using plain radiographs from 1 week to 12 months after injury with an interval of 2 to 10 weeks. Computed tomography was performed in 2 studies. In 4 of 11 included studies, teriparatide was injected after surgical fixation, of which location was pertrochanteric in 2, neck of the femur 1, and distal femur in 1.
The healing rate of pelvis fractures was significantly higher in the teriparatide treatment group, comparing to the control group (pubis; 100% vs. 9.1%, P<0.001 and sacrum; 100% vs. 20%, P<0.001) 8 weeks after trauma (Table 2).[829] However, the healing rate in pertrochanteric or neck fractures of the femur did not show significant differences between the groups 3, 6, and 12 months after surgery.[3031] Time to union was significantly shorter in the pubis (7.8 weeks vs. 12.6 weeks, P<0.001) and in the distal femur (28.7 weeks vs. 41.9 weeks, P=0.001), but was controversial in pertrochanteric femur.[832] In distal radius fractures, the healing time was significantly different between the teriparatide (20 µg) and the placebo groups (7.4 weeks vs. 9.1 weeks, P=0.01), but not different between the teriparatide (40 µg) and the placebo groups (8.8 weeks vs. 9.1 weeks, P=0.52).[28]
Visual analog scale (VAS) of pain was significantly reduced in the teriparatide group, comparing to the control group in pelvis and pertrochanteric femur.[8293033] However, the VAS pain score did not show significant differences between the groups in fractures of neck of the femur, proximal humerus, and distal radius.[283134] In spine fractures, teriparatide showed various effects on pain relief per weeks after trauma.[3536] Physical performances including Timed Up-and-Go (TUG) test and time to mobilization were significantly improved in the teriparatide group with fractures of the pelvis and pertrochanteric femur[29303133] but, other function scores such as disabilities of the arm, shoulder, and hand (DASH) and grip strength revealed no differences in fractures of proximal humerus and distal radius after teriparatide injection.[2834]
In pelvic bone, all of the 2 studies concluded that the mean time to fracture healing was significantly shorter in the PTH treatment group.[829] The mean time to mobilization was also found to be reduced in the treatment group. In 2 studies with pertrochanteric fractures,[3033] teriparatide was associated with less pain and a shorter time to functional recovery. With regard to the radiographic healing, one study reported that there was a significant difference between the teriparatide-treated group and the placebo group but the other study reported no difference between the teriparatide-treated group and the risedronate-treated group. In a randomized placebo-controlled trial on femoral neck fracture, teriparatide did not seem to improve radiographic signs of fracture healing or decrease pain compared with the placebo.[31] In comminuted distal femur fractures in elderly, teriparatide was effective in bridging callus formation and bone union.[32]
In upper-extremities, 2 well-designed clinical trials were identified. One study focusing on proximal humerus fractures showed that teriparatide did not enhance fracture healing and not decrease pain scale at early recovery period within 3 months.[34] In contrast, the other study on distal radius fractures revealed that teriparatide (20 µg) shortened the time of healing and their post hoc subgroup analysis showed a positive dose-related effect on early callus formation.[2837]
When the effect of teriparatide and risedronate on back pain was investigated, there was no difference in reduction of pain numeric scales in one study.[35] However, patients receiving teriparatide had greater skeletal benefit than those receiving risedronate. In contrast, the other study showed significant reduction of pain numerical scale within 8 or 12 weeks after trauma.[36]
No difference in the incidence of adverse events was noted in all of studies.
Osteoporotic fractures have gained increased attention in terms of poor functional recovery, complicated comorbidities, and high economic burden.[3839] Despite advances in surgical technique and implant design, age-related decreases in bone regenerative capacities and poor bone stock remains problematic.[33]
Substantial efforts to improve fracture healing yielded further medication such as bisphosphonates, bone morphogenetic proteins, and PTH.[183740] In particular, a potential impact of PTH on human fracture repair was demonstrated in various animal and human studies.[16183741424344] In an animal model with two hundred and seventy male Sprague-Dawley rats undergoing closed femoral fractures, daily administered PTH (1-34)-treated group showed significant increases over the controls with respect to torsional strength, stiffness, bone mineral content, and cartilage volume by day 21.[41] Andreassen et al.[45] compared the effect of intermittent PTH 1-34 on fracture healing in 2 to 3-month-old rats with aged (21-month-old) rats. This study noted that a reduction in the number of osteoprogenitor cells and/or a diminished rate of maturation of pre-osteoblasts to osteoblasts is correlated with old age and questioned whether older animals would respond to PTH as robustly as younger animals. Their findings showed that both old and young PTH-treated animals had achieved maximum callus volumes by day 21 after fracture and PTH enhanced ultimate load of the fractures by 160% at 21 days of healing and by 270% at 56-days, in comparison with controls.
Clinical evidences for the use of teriparatide in fracture healing were searched on recent 2 meta-analysis articles. Lou et al. [46] identified 5 RCTs (251 patients) comparing teriparatide to placebo, no treatment, or comparator interventions in the osteoporotic patients. Patients treated with teriparatide therapy had a significant shorter healing time compared with those in the control group. Stratified analysis showed that the lower limb group had significant shorter healing time, but upper limb group did not. However, the other meta-analysis recruiting a total of 380 patients from 5 RCTs yielded that there was no significant effectiveness with regards to time-to-union, union rate, and reduction in pain.[47] These data might be underpowered to detect any difference, considering limited number of the included studies. Interpretation also needs to be cautioned due to discrepancies in study design.
In current review, clinical trials or case-controlled studies on treatment of osteoporotic fractures using PTH were recruited, including 4 retrospective controlled series, 6 RCTs, and 1 retrospective post hoc subgroup analysis. The use of teriparatide yielded positive effects on radiographic bone healing in 6 studies, but was not associated with better radiographic outcome in 3. In terms of functional recovery, teriparatide injection was related with decrease in pain or shorter time to mobilization in 6 studies, but not related with pain numerical scale and mobility in 3. Our findings suggest that teriparatide provide selective advantages to fracture healing or functional recovery in the management of osteoporotic fractures. Teriparatide did not necessarily guarantee successful recovery of osteoporotic fractures. We think that there are many other factors that influence patients' outcome. Moreover, in cases of surgically treated fractures, quality of surgery including anatomical approach, reduction techniques, and selection of fixation modality is prerequisite for obtaining successful healing. The use of teriparatide, thereafter, should be considered as one of supplemental options.
Some limitations should be described in this study. Although we extracted data only from well controlled studies, interpretation needs to be cautioned. Two RCTs designed the control regimen as not placebo, but risedronate.[3035] A trial on pubic bone fracture had some methodological shortcomings as the investigator was not independent upon determining whether or not to include a patient.[8] In a placebo-controlled trial showing enhanced fracture healing of distal radius, the primary outcome using the higher teriparatide dose was not significant.[28] A similar trial on proximal humerus fractures depended only on statement of radiologists.[34] Second, our findings were limited by heterogeneity of the study design, study population, fracture location, and primary measured outcome. There is still no consensus regarding which outcome variables should be evaluated, and when or how the measurement is done. Plain radiographs obtained at the selected intervals may be too crude for evaluation of fracture healing. Third, due to the limited available data, we included the evidence for PTH (1-84) in our analysis even if this drawback confined to only 1 clinical trial. Lastly, case series enforcing our evidence was not included. A series of 29 patients with unstable pertrochanteric fractures reported that rates of lag screw sliding, femoral shortening, and varus collapse were all reduced in the teriparatide-treated group.[48]
In conclusion, the effect of teriparatide on fracture healing and functional recovery of osteoporotic patients remains uncertain. However, there was no evidence that the use of teriparatide had been harmful in management of osteoporotic fractures and no difference in the incidence of adverse events in all of included studies. A better understanding of the role of teriparatide on fractures healing requires greater evidences from large volume prospective studies. According to their results, further studies regarding different dosage, regimen, and treatment period may be investigated in the near future. Teriparatide is still an attractive option that may contribute to fracture healing and functional recovery in osteoporotic fracture patients.
Figures and Tables
Table 1
Summary of the published studies with intermittent parathyroid hormone administration in osteoporotic fractures

PTH, parathyroid hormone; RCT, randomized clinical trial; TPTD, teriparatide; Ca, calcium; Vit D, vitamin D; CT, computed tomography; VAS, visual analog scale; TUG, Timed Up-and-Go; SF-12, short-form 12 health survey; DASH, disabilities of the arm, shoulder, and hand; PRWE, patient-related wrist evaluation.
References
1. Murray TM, Rao LG, Divieti P, et al. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr Rev. 2005; 26:78–113.

2. Kurland ES, Cosman F, McMahon DJ, et al. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab. 2000; 85:3069–3076.

3. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014; 370:412–420.

4. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005; 16:510–516.

5. Lindsay R, Scheele WH, Neer R, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004; 164:2024–2030.

6. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001; 344:1434–1441.

7. Orwoll ES, Scheele WH, Paul S, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res. 2003; 18:9–17.

8. Peichl P, Holzer LA, Maier R, et al. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am. 2011; 93:1583–1587.

9. Prevrhal S, Krege JH, Chen P, et al. Teriparatide vertebral fracture risk reduction determined by quantitative and qualitative radiographic assessment. Curr Med Res Opin. 2009; 25:921–928.

10. Prince R, Sipos A, Hossain A, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res. 2005; 20:1507–1513.

11. Binder EF, Brown M, Sinacore DR, et al. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004; 292:837–846.

12. Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000; 55:M498–M507.

13. Kawaguchi H, Oka H, Jingushi S, et al. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J Bone Miner Res. 2010; 25:2735–2743.

14. Sharrard WJ. A double-blind trial of pulsed electromagnetic fields for delayed union of tibial fractures. J Bone Joint Surg Br. 1990; 72:347–355.

15. Wright JG, Einhorn TA, Heckman JD. Grades of recommendation. J Bone Joint Surg Am. 2005; 87:1909–1910.

16. Komrakova M, Stuermer EK, Werner C, et al. Effect of human parathyroid hormone hPTH (1-34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone. 2010; 47:480–492.

17. Lina IA, Puvanesarajah V, Liauw JA, et al. Quantitative study of parathyroid hormone (1-34) and bone morphogenetic protein-2 on spinal fusion outcomes in a rabbit model of lumbar dorsolateral intertransverse process arthrodesis. Spine (Phila Pa 1976). 2014; 39:347–355.

18. Morimoto T, Kaito T, Kashii M, et al. Effect of intermittent administration of teriparatide (parathyroid hormone 1-34) on bone morphogenetic protein-induced bone formation in a rat model of spinal fusion. J Bone Joint Surg Am. 2014; 96:e107.

19. Mancilla EE, Brodsky JL, Mehta S, et al. Teriparatide as a systemic treatment for lower extremity nonunion fractures: a case series. Endocr Pract. 2015; 21:136–142.

20. Chiang CY, Zebaze RM, Ghasem-Zadeh A, et al. Teriparatide improves bone quality and healing of atypical femoral fractures associated with bisphosphonate therapy. Bone. 2013; 52:360–365.

21. Miyakoshi N, Aizawa T, Sasaki S, et al. Healing of bisphosphonate-associated atypical femoral fractures in patients with osteoporosis: a comparison between treatment with and without teriparatide. J Bone Miner Metab. 2015; 33:553–559.

22. World Health Organization. Guidelines for preclinical evaluation and clinical trials in osteoporosis. Geneva, CH: World Health Organization;1998.
23. Brown JP, Josse RG. 2002 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002; 167:S1–34.
24. Cerocchi I, Ghera S, Gasbarra E, et al. Fragility fractures: the clinical pathway. Aging Clin Exp Res. 2013; 25:Suppl 1. S43–S45.

25. Ralston SH, Fraser J. Diagnosis and management of osteoporosis. Practitioner. 2015; 259:15–19. 2
26. Yoo JH, Moon SH, Ha YC, et al. Osteoporotic fracture: 2015 position statement of the Korean society for bone and mineral research. J Bone Metab. 2015; 22:175–181.

27. National Institute for Health and Care Excellence. Osteoporosis: assessing the risk of fragility fracture. 2012. 2012 Oct 16. Available from: https://www.nice.org.uk/guidance/CG146.
28. Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010; 25:404–414.

29. Ha YC, Park YG, Nam KW, et al. Trend in hip fracture incidence and mortality in Korea: a prospective cohort study from 2002 to 2011. J Korean Med Sci. 2015; 30:483–488.

30. Aspenberg P, Malouf J, Tarantino U, et al. Effects of teriparatide compared with risedronate on recovery after pertrochanteric hip fracture: results of a randomized, active-controlled, double-blind clinical trial at 26 weeks. J Bone Joint Surg Am. 2016; 98:1868–1878.

31. Bhandari M, Jin L, See K, et al. Does teriparatide improve femoral neck fracture healing: results from a randomized placebo-controlled trial. Clin Orthop Relat Res. 2016; 474:1234–1244.

32. Song HK, Kim SJ, Lee JH, et al. Intermittent parathyroid hormone treatment for stimulation of callus formation in elderly patients. J Korean Fract Soc. 2012; 25:295–299.

33. Huang TW, Chuang PY, Lin SJ, et al. Teriparatide improves fracture healing and early functional recovery in treatment of osteoporotic intertrochanteric fractures. Medicine (Baltimore). 2016; 95:e3626.

34. Johansson T. PTH 1-34 (teriparatide) may not improve healing in proximal humerus fractures. A randomized, controlled study of 40 patients. Acta Orthop. 2016; 87:79–82.

35. Hadji P, Zanchetta JR, Russo L, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int. 2012; 23:2141–2150.

36. Tsuchie H, Miyakoshi N, Kasukawa Y, et al. The effect of teriparatide to alleviate pain and to prevent vertebral collapse after fresh osteoporotic vertebral fracture. J Bone Miner Metab. 2016; 34:86–91.

37. Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop. 2010; 81:234–236.

38. Orive M, Aguirre U, Garcia-Gutierrez S, et al. Changes in health-related quality of life and activities of daily living after hip fracture because of a fall in elderly patients: a prospective cohort study. Int J Clin Pract. 2015; 69:491–500.

39. Vergara I, Vrotsou K, Orive M, et al. Factors related to functional prognosis in elderly patients after accidental hip fractures: a prospective cohort study. BMC Geriatr. 2014; 14:124.

40. Seo JB, Yoo JS, Ryu JW, et al. Influence of early bisphosphonate administration for fracture healing in patients with osteoporotic proximal humerus fractures. Clin Orthop Surg. 2016; 8:437–443.

41. Alkhiary YM, Gerstenfeld LC, Krall E, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg Am. 2005; 87:731–741.

42. Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999; 14:960–968.

43. Barnes GL, Kakar S, Vora S, et al. Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J Bone Joint Surg Am. 2008; 90:Suppl 1. 120–127.

44. Bashutski JD, Eber RM, Kinney JS, et al. Teriparatide and osseous regeneration in the oral cavity. N Engl J Med. 2010; 363:2396–2405.

45. Andreassen TT, Fledelius C, Ejersted C, et al. Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop Scand. 2001; 72:304–307.

46. Lou S, Lv H, Wang G, et al. The effect of teriparatide on fracture healing of osteoporotic patients: a meta-analysis of randomized controlled trials. Biomed Res Int. 2016; 2016:6040379.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download