Abstract
Background
In this study, we evaluated the prevalence of osteoporosis, risk factors associated with osteoporosis, and health-related quality of life (HRQOL) in clinically stable chronic obstructive pulmonary disease (COPD) patients.
Methods
A total of 1,081 COPD patients were recruited from the Korea National Health and Nutrition Examination Survey (KNHANES) from July 2008 to May 2011. Bone mineral densities at the lumbar spine, femoral neck, and total proximal femur were measured using dual energy X-ray absorptiometry. HRQOL was assessed using the EuroQOL-5 dimensions (EQ-5D) questionnaire. To identify factors associated with osteoporosis and HRQOL in patients with COPD, multivariate regression analyses was performed.
Results
Of the 1,081 COPD patients, 191 (17.7%) were diagnosed with osteoporosis. There were significant differences in age, sex, smoking status, education level, house income, and body mass index (BMI) between the osteoporotic and non-osteoporotic groups. COPD patients with osteoporosis had significantly lower EQ-5D scores than the controls. In multivariate analyses, older age (odds ratio [OR]=1.10, P<0.001) was risk factor for osteoporosis. And patients of male sex (OR=0.06, P<0.001), high house income (OR=0.75, P=0.045), and high BMI (OR=0.74, P<0.001) were less likely to have osteoporosis. In addition, osteoporosis was associated with poor HRQOL (β=−0.21, P=0.023).
Osteoporosis is a systemic skeletal disorder that is characterized by low bone mass and microarchitectural changes that increase bone fracture risk.[1] Fracture risk depends on bone strength, which is determined by bone mineral density (BMD) and bone quality.[2] Osteoporosis is an important public health problem worldwide, because osteoporotic fractures are associated with increased mortality, functional decline, loss of quality of life (QOL), and a need for institutionalization in older individuals.[3]
Osteoporosis is one of the major systemic comorbidity of chronic obstructive pulmonary disease (COPD).[4] The prevalence of osteoporosis in COPD patients is 2-fold to 5-fold higher than in age-matched healthy control subjects.[5] The reported prevalence ranges widely from 4% to 59%, depending on the diagnostic methods used, the population studied, and the severity of the underlying respiratory disease.[167] Most studies on osteoporosis in COPD patients have included patients with severe disease who are predominantly treated with glucocorticoids. Understanding the risk factors for osteoporosis in COPD has been difficult due to the therapeutic use of systemic glucocorticoids.[8] The prevalence of osteoporosis in mild or asymptomatic COPD patients has not been well studied. In addition, there are few studies on the risk factors for osteoporosis in COPD patients with low exposure to steroids. It is important to identify the prevalence and risk factors of osteoporosis, because it is often underdiagnosed and associated with poor health-related QOL (HRQOL) in COPD patients.[9]
Therefore, in this study, we analyzed the prevalence of osteoporosis and the risk factors associated with osteoporosis in clinically stable or asymptomatic COPD patients, based on the national database of information on medical utilization developed by the Korea National Health and Nutrition Examination Survey (KNHANES). In addition, we examined the association between HRQOL and osteoporosis in COPD patients.
The KNHANES is a nationwide representative cross-sectional survey of the Korean population with a clustered, multistage, stratified, and rolling sampling design.[10] It consists of three sections: a health interview, health examination, and nutrition survey. The health interview section focuses on the respondents' socioeconomic status, demographics, health behaviors, and QOL. The health examination section focuses on the diagnosis of chronic disease by screening or examining health status. Finally, the nutrition section focuses on surveying food and nutrition behaviors. We collected data of 21,303 adults older than 50 years of age from the KNHANES from July 2008 to May 2011 (Fig. 1). We excluded participants who did not have results of pulmonary function tests (PFTs) and dual energy X-ray absorptiometry (DXA).
A diagnosis of COPD was established based on PFTs using the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.[11] According to the GOLD criteria, individuals with a ratio of forced expiratory volume in 1 second (FEV1) to functional vital capacity (FVC) <0.7 were considered to have COPD. Spirometry was performed according to the American Thoracic Society/European Respiratory Society criteria for standardization.[12] We classified severity of airflow limitation according to the GOLD criteria (stage I, mild FEV1 ≥80%; stage II, moderate FEV1 50–80%; stage III, severe FEV1 30–50%; stage IV, very severe FEV1 <30% predicted or FEV1 <50% predicted plus chronic respiratory failure).[11]
BMD at the lumbar spine, femoral neck, and total proximal femur were measured using DXA (Hologic Inc., Bedford, MA, USA). According to the World Health Organization study group, the diagnosis of osteoporosis is based on T-score thresholds.[13] T-scores at or above −1.0 are considered normal, those between −1.0 and −2.5 are considered osteopenia, and those at or below −2.5 are considered osteoporosis.[13] BMD screening was performed from July 2008 to May 2011 only in participants who were 50 and older or women in menopause.
HRQOL was assessed using the EuroQOL-5 dimensions (EQ-5D) questionnaire, which generates assessment scores across five dimensions of health, mobility, self-care, usual activity, pain/discomfort, and anxiety/depression.[14] Responses in each dimension were divided into three categories: no problem, moderate problem, or extreme problem.[14] Average scores on the EQ-5D index were calculated to assess HRQOL, which is a preference-based health status index. Because the preference weights of Koreans are quite different from those of Caucasians, we used Korean-specific preference weights to generate the EQ-5D index scores.[15] Average EQ-5D scores ranged from −0.17 to 1, where 1 indicates no problem in any of the five dimensions.[15] The average score of the EQ-5D index was calculated to assess the QOL of COPD patients.
House income was categorized into four groups by quartile. The lowest group was the 25th percentile of sex- and age-specific income distributions. Body weight and height were measured in light clothing with no shoes, and body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Information regarding comorbidities including hypertension, diabetes, hyperlipidemia, depression, and malignancy as potential confounding factors were examined using the health interview survey.
All statistical analyses were made considering sample weights that were constructed based on the survey design, survey non-response and post-stratification. Continuous data are expressed as means±standard deviations and categorical data as numbers with percentages. Characteristics between osteoporosis and non-osteoporosis groups were compared using independent Student's t-tests for continuous parameters or χ2 tests for categorical parameters. To identify factors contributing to osteoporosis and HRQOL in patients with COPD, a multivariate regression was performed. Specifically, multivariate logistic analysis was performed to identify factors contributing to the prevalence of osteoporosis and beta regression was conducted to examine the factors associated with the HRQOL among patients with COPD by adjusting to normal distributions. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. The Institutional Review Board (IRB) of the Korea Centers for Disease Control and Prevention reviewed and approved the KNHANES survey annually and the IRB approval numbers were 2008–04EXP-01-C, 2009– 01CON-03–2C, 2010–02CON-21-C, and 2011–02CON-06-C. All of the data can be downloaded from the official website of the KNHANES (http://knhan-es.cdc.go.kr/). These data are open to the public after completing a designated registration process for access.
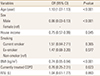
The clinical characteristics of the 1,081 COPD patients according to osteoporosis status are shown in Table 1. Of the 1,081 patients, 191 (17.7%) were diagnosed with osteoporosis. There were significant differences in age, sex, smoking status, socioeconomic status, and BMI between the osteoporosis and non-osteoporosis groups. The osteoporosis group had lower lumbar spine, total femur, and femoral neck BMD, and lower T-scores than the non-osteoporosis group. The history of prescribing COPD treatment did not differ between the two groups (1.1% vs. 1.4%, P=0.730). FVC and FEV1 were lower in the osteoporosis group than in the non-osteoporosis group. However, the prevalence of osteoporosis by severity of COPD according to airflow limitation did not significantly differ between the groups (P=0.860) (Fig. 2).
Table 2 shows the comparison of the HRQOL by EQ-5D questionnaires between the osteoporosis and non-osteoporosis groups. The total EQ-5D score was significantly lower in the osteoporosis group than in the non-osteoporosis group (0.84±0.02 vs. 0.92±0.01, P<0.001). More patients in the osteoporosis group answered that they had some problems or had problems in all of the survey dimensions.
Table 3 shows the multivariate logistic analysis for evaluating risk factors associated with osteoporosis in COPD patients. The risk for osteoporosis increased 1.1 times more with increased age (p<0.001). On the other hand, patients of male sex (odds ratio [OR]=0.06, P<0.001), high house income (OR=0.75, P=0.045), and high BMI (OR=0.74, P<0.001) were less likely to have osteoporosis. FEV1 was not an independent risk factor for osteoporosis (OR=1.04, P=0.893).
To evaluate factors associated with HRQOL in patients with COPD, beta regression analysis was performed (Table 4). Aging contributed negatively to HRQOL (β=−0.01, P=0.031). HRQOL increased by 0.09 with increased house income (P=0.007). The severity of airflow limitation showed no significant association with HRQOL, except for severity IV, which deteriorated HRQOL by 0.8 compared to severity I (P=0.004). Osteoporosis was associated with poor HRQOL (β=−0.21, P=0.023).
The prevalence of osteoporosis in our study was approximately 17.7%. This result was lower than the prevalence in other COPD studies and higher than that in the general population.[6161718] Studies have shown that patients with COPD usually have low BMD, impaired bone quality, and low bone turnover.[4] The mechanisms by which osteoporosis occurs in COPD patients are not yet known. However, clinical evidence has indicated that systemic inflammation, pulmonary dysfunction, and glucocorticoid use are associated with risk factors.[4] This study used KNHANES data; thus these patients were not physician-diagnosed with COPD, which suggests that clinically stable or asymptomatic patients were included in this dataset. In addition, the severity of airway obstruction in this study was mild (mean FEV1 of all patients was ~76-77%). The mean age was also relatively lower than in other studies. These findings may have affected the overall prevalence.
In this study, a low FEV1 was not an independent risk factor for osteoporosis. However, the prevalence of osteoporosis increased slightly with increasing airflow limitation although not statistically significant (stage I, 17.8% vs. stage IV, 28.6%; P=0.860) (Fig. 2). The correlation between pulmonary function and BMD has been inconsistent in previous studies. Several studies have reported that low FEV1 increased the risk for osteoporosis in COPD patients.[192021] Patients with severe airflow limitation (predicted FEV1 <30%) are particularly at risk for developing osteoporosis.[20] Although the exact mechanism is unclear, systemic inflammation, low physical activity levels, and corticosteroid-induced loss of bone mass are potential explanatory mechanisms.[202223] The correlation between BMD and FEV1 has also been demonstrated in the general population. Jeon et al.[17] reported that FVC and FEV1 are associated with BMD in healthy nonsmoking premenopausal women but not in postmenopausal women. Lee et al.[24] found that FEV1 was negatively correlated with lumbar spine BMD but not femur neck or total hip BMD in middle-aged Korean men. Both studies were conducted using KNHANES data, as in this study. Our study population was not physician-diagnosed COPD patients, so there was a relatively higher level of physical activity and lower exposure to corticosteroids than in patients in other COPD studies. This may explain the lack of association between measures of lung function and bone mass.
Body weight loss and cachexia are frequently found in COPD, particularly at advanced levels of airflow limitation.[25] Low BMI has been shown to predict osteoporosis among COPD patients.[51926] In this study, low BMI independently increased osteoporosis risk. This finding is consistent with other studies. Although the mechanism is unclear, there are several obvious points: body weight impacts both bone turnover and bone density; therefore, it is a most important determinants of BMD and fracture risk.[27] Both fat and lean mass contribute to this relationship. In addition, cachexia in severe COPD has been attributed to systemic inflammation, with increased levels of cytokines and oxidative stress.[28]
HRQOL in COPD patients can be assessed by generic and disease-specific instruments. EQ-5D is a generic instrument for assessing health status. It is a brief, simple, and cost-effective measure.[14] Studies have shown that generic instruments such as the EQ-5D or the Short Form 12 are suitable for measuring HRQOL for both stable status and acute exacerbations in COPD patients.[29] The EQ-5D score is also capable of differentiating among patients with COPD severity, as defined by GOLD criteria.[30] Dimensions of mobility, self-care, and usual activity in particular are significantly associated with pulmonary function.[30] In our study, the EQ-5D score of the osteoporosis group was significantly lower than that of the non-osteoporosis group. The dimensions of mobility and usual activity differed strongly between the two groups.
The association between osteoporosis and poor HRQOL in COPD patients is well established in the general population. The main factor for low HRQOL in patients with osteoporosis is osteoporosis-related fractures, particularly vertebral fractures.[3132] However, there is a paucity of data regarding osteoporosis with HRQOL in COPD patients. Osteoporosis-related fractures may play an important role in poor HRQOL in COPD patients.[3334] Poor HRQOL, as assessed by EQ-5D, was significantly associated with osteoporosis. We could not collect data on the presence of fractures, but we speculate that fracture status and HRQOL may be related. In the future, it will be necessary to investigate whether treatment of osteoporosis can improve the QOL in COPD patients.
This study is significant in that it analyzed the prevalence of osteoporosis and HRQOL in COPD patients through a large national database. However, there were some limitations in this study. First, this study is cross-sectional study, therefore, it does not allow definite determintation of causal associations. Second, the subjects were only diagnosed by pulmonary function rather than by hospital physicians. Therefore, patients who do not have COPD may have also been included, because asthma patients may also have decreased pulmonary function, and in some older people, pulmonary function is asymptomatically decreased. Third, COPD is mostly caused by smoking in Korea, but many non-smokers were included in the dataset. Finally, information regarding possible risk factors such as systemic corticosteroid use was unavailable during our study. Instead, a history of prescribing COPD treatment was suggested, and it was assumed that this treatment involved steroids.
In conclusion, the prevalence of osteoporosis in mild or asymptomatic COPD patients was relatively lower than that in moderate to severe COPD patients. Older age, female sex, low household income, and low BMI increased the risk for osteoporosis in patients with mild COPD. Patients with these risk factors should be advised to check BMD regularly. Osteoporosis is independently associated with poor HRQOL; therefore, if osteoporosis is diagnosed, aggressive treatment is necessary.
Figures and Tables
Fig. 1
Flow chart of the study population. COPD, chronic obstructive pulmonary disease; BMD, body mineral density.
Fig. 2
Prevalence of osteoporosis according to the severity of chronic obstructive pulmonary disease. Stage I: forced expiratory volume in 1 second (FEV1)≥80%, stage II: FEV1=50–80%, stage III: FEV1=30–50%, stage IV: FEV1<30% (P=0.860).
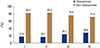
Table 1
Clinical characteristics of study subjects with and without osteoporosis
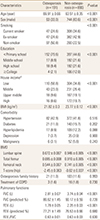
a)House income was categorized into four groups based on its quartiles by sex and age.
The data is presented as mean±standard deviation or number (%).
BMI, body mass index; BMD, body mineral density; COPD, chronic obstructive pulmonary disease; FVC, functional vital capacity; FEV1, forced expiratory volume in 1 second.
ACKNOWLEDGMENTS
The authors would like to thank Hayon Michelle Choi for her contributions to the statistical analysis.
References
1. Lehouck A, Boonen S, Decramer M, et al. COPD, bone metabolism, and osteoporosis. Chest. 2011; 139:648–657.

2. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001; 285:785–795.
3. Haentjens P, Magaziner J, Colón-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010; 152:380–390.

4. Inoue D, Watanabe R, Okazaki R. COPD and osteoporosis: links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis. 2016; 11:637–648.

5. Bolton CE, Ionescu AA, Shiels KM, et al. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170:1286–1293.

6. Ferguson GT, Calverley PMA, Anderson JA, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the TOwards a Revolution in COPD Health study. Chest. 2009; 136:1456–1465.

7. Sin DD, Man JP, Man SF. The risk of osteoporosis in Caucasian men and women with obstructive airways disease. Am J Med. 2003; 114:10–14.

8. Putcha N, Drummond MB, Wise RA, et al. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Semin Respir Crit Care Med. 2015; 36:575–591.

9. Wen CP, Levy DT, Cheng TY, et al. Smoking behaviour in Taiwan, 2001. Tob Control. 2005; 14:Suppl 1. i51–i55.

10. Ministry of Health & Welfare. Korea Centers for Disease Control & Prevention. Korea health statistics 2010-2012: Korea national health and nutrition examination survey (KNHANES V) 2010-2012. Seoul: Ministry of Health & Welfare;2011.
11. Global Initiative for Chronic Obstructive Lung Disease. The global strategy for the diagnosis, management and prevention of COPD. 2017. cited by 2017 October 1. Available from: http://goldcopd.org.
12. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.

13. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994; 843:1–129.
14. Pickard AS, Wilke C, Jung E, et al. Use of a preference-based measure of health (EQ-5D) in COPD and asthma. Respir Med. 2008; 102:519–536.

15. Lee YK, Nam HS, Chuang LH, et al. South Korean time trade-off values for EQ-5D health states: modeling with observed values for 101 health states. Value Health. 2009; 12:1187–1193.

16. Franco CB, Paz-Filho G, Gomes PE, et al. Chronic obstructive pulmonary disease is associated with osteoporosis and low levels of vitamin D. Osteoporos Int. 2009; 20:1881–1887.

17. Jeon YK, Shin MJ, Kim WJ, et al. The relationship between pulmonary function and bone mineral density in healthy nonsmoking women: the Korean National Health and Nutrition Examination Survey (KNHANES) 2010. Osteoporos Int. 2014; 25:1571–1576.

18. Park EJ, Joo IW, Jang MJ, et al. Prevalence of osteoporosis in the Korean population based on Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2011. Yonsei Med J. 2014; 55:1049–1057.

19. Watanabe R, Tanaka T, Aita K, et al. Osteoporosis is highly prevalent in Japanese males with chronic obstructive pulmonary disease and is associated with deteriorated pulmonary function. J Bone Miner Metab. 2015; 33:392–400.

20. Vrieze A, de Greef MH, Wijkstra PJ, et al. Low bone mineral density in COPD patients related to worse lung function, low weight and decreased fat-free mass. Osteoporos Int. 2007; 18:1197–1202.

21. Kjensli A, Falch JA, Ryg M, et al. High prevalence of vertebral deformities in COPD patients: relationship to disease severity. Eur Respir J. 2009; 33:1018–1024.

22. Gan WQ, Man SF, Senthilselvan A, et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004; 59:574–580.

23. Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005; 171:972–977.

24. Lee JH, Hong AR, Kim JH, et al. Amount of smoking, pulmonary function, and bone mineral density in middle-aged Korean men: KNHANES 2008-2011. J Bone Miner Metab. 2017; DOI: 10.1007/s00774-017-0811-1.

25. Vestbo J, Prescott E, Almdal T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006; 173:79–83.

26. Graat-Verboom L, Spruit MA, van den Borne BE, et al. Correlates of osteoporosis in chronic obstructive pulmonary disease: an underestimated systemic component. Respir Med. 2009; 103:1143–1151.

28. Remels AH, Gosker HR, Langen RC, et al. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol (1985). 2013; 114:1253–1262.

29. Menn P, Weber N, Holle R. Health-related quality of life in patients with severe COPD hospitalized for exacerbations - comparing EQ-5D, SF-12 and SGRQ. Health Qual Life Outcomes. 2010; 8:39.

30. Rutten-van Mölken MP, Oostenbrink JB, Tashkin DP, et al. Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages? Chest. 2006; 130:1117–1128.

31. Kwon HY, Ha YC, Yoo JI. Health-related quality of life in accordance with fracture history and comorbidities in Korean patients with osteoporosis. J Bone Metab. 2016; 23:199–206.

32. Jung HJ, Park YS, Seo HY, et al. Quality of life in patients with osteoporotic vertebral compression fractures. J Bone Metab. 2017; 24:187–196.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download