Abstract
Background
Since 1991 many studies evaluated the link between cardiovascular diseases and osteoporosis, two age-related conditions, but the main common pathologic pathway has not been determined yet. The histological similarity between arterial calcified plaque and bone matrix and involvement of similar cells and mediators provide a special field of research. Therefore in the present study, we aimed to evaluate the relationship between coronary artery calcium score (CACS) as a surrogate marker of atherosclerosis and bone mediators and parameters in postmenopausal women.
Methods
Eleven postmenopausal women who had CACS higher than 80 were enrolled into the study and underwent bone densitometry. In addition, their serum and urine samples were taken for measuring osteoprotegerin, osteocalcin, and β cross laps. Patients' 10-year probability of fracture was calculated by the World Health Organization fracture-risk assessment tool (FRAX).
Results
The regression analysis of our results showed the association between CACS and OC (std β=0.66, 95% confidence interval [CI] 5.47-72.27, P=0.027), femoral bone density (std β=−0.6, 95% CI -6864.34-14.27, P=0.05) and T-score (std β=−0.6, 95% CI −773.08-1.28, P=0.05) which remained significant after adjustment for age, weight, years since menopause and body mass index. No association was found between CACS and osteoprotegerin, spinal bone density and FRAX score.
Conclusions
In conclusion, this pilot study with small sample size showed the potential association between CACS and osteocalcin, femoral bone density and T-score. However, the relationship between CACS and osteoprotegerin, receptor activator of nuclear factor-kappa B ligand, FRAX score and other bone parameters remain to be clarified in larger sample size studies.
Special attention has been paid to the link between atherosclerosis and osteoporosis since 1991 and it is assumed to be regulated by biological mechanisms. Both diseases are considered to be associated with aging. Although at the first glance osteoporosis and atherosclerosis seem to be independent, atherosclerotic plaques and bone tissue have similarities and the process of vascular calcification is mediated by cells and mediators involved in bone formation.[1] In this regard several common inflammatory and proinflammatory mediators including, bone markers and cytokines including interleukin (IL)-1, IL-6, IL-11, IL-12, IL-15, IL-17, tumor necrosis factor-alpha (TNF-α), osteoprotegerin, bone morphogenetic proteins (BMPs), C-reactive protein (CRP) have been suggested but still the main common causative agent or pathway is not fully understood.[1] Histological findings demonstrated resorptive and remodeling sites in atherosclerotic plaques similar to bone. Actually these plaques have abundant numbers of different types of inflammatory cells inducing several types of mediators. Vascular endothelium and vascular smooth muscle cells produce osteoprotegerin and receptor activator of nuclear factor-kappa B ligand (RANKL) that is expressed in atherosclerotic lesions.[2] Osteoprotegerin acts as a member of TNF-α superfamily and inhibits osteoclast differentiation and bone resorption by binding to RANKL. RANKL is involved in vascular calcification by inducing osteoclast formation in vascular smooth muscle cells and counteracts with osteoprotegerin.[3] The counteraction of osteoprotegerin with RANKL regulates bone resorption. In addition osteoprotegerin controls bone pathological conditions including bone metabolism and inflammation. Human studies indicated the possible direct association between osteoprotegerin and severity of coronary artery disease (CAD).[1] In line with the evidence of involvement of osteoprotegerin, there are recent evidences which highlight the role of osteocalcin in osteoporosis and cardiovascular disease (CVD). Osteocalcin is a bone matrix protein mainly produced by osteoblasts which is involved in bone mineralization and formation.[4] Osteoblasts like cells in the vasculature are responsible for secreting osteocalcin and calcifying vascular cells.[5]
There are recent evidences of the involvement of osteocalcin in CAD.
One of the surrogate markers of atherosclerosis is coronary artery calcium (CAC) score (CACS) which can predict cardiovascular risk.[6] Data regarding the correlation between CACS and bone metabolism are still paradox. Accordingly and in order to find out the relationship between CACS as a surrogate marker of atherosclerosis and bone biomarkers as well as bone density and other bone specific information, we conducted a pilot study to provide presumptive data for more comprehensive assessment. Our study is the first one which evaluated this association in postmenopausal women who had CACS higher than 80 which indicates the average risk of atherosclerosis and higher.
Eleven postmenopausal women who underwent computed tomography scanning with (Dual Source Flash-128 slice) and had CACS higher than 80 were enrolled in the study. Based on the categories of CACS which was defined in previous studies,[7] we selected CACS ≥80 which is considered as average risk of coronary artery events and higher.
The eligible patients were referred to the Diabetic Research Center for measuring bone density. Each subject underwent bone densitometry by dual energy X-ray absorptiometry (DXA; Hologic Inc., Bedford, MA, USA) at femur neck and lumbar vertebrae respectively. Blood and urine samples were obtained from study participants. All patients profile was evaluated and recorded including age, menopause state, smoking, alcohol consumption, history of fracture, parents fracture, and past medical history. Patients with history of cancer, diabetes, acute infection, endocrinologic disorders, use of special medications such as hormones, corticosteroids, gonadotropin releasing hormone (GnRH) analogs, anticonvulsant drugs, heparin, aluminium containing antacids, thyroid hormones were excluded. The study was conducted in Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences after authorization by ethics committee of the institute. All patients signed the written informed consent after receiving thorough information about the study.
Blood and urine samples were collected. Blood samples were centrifuged at 10,000 g for 10 min and kept in a fridge at −70℃ until analysis. Serum concentration of osteoprotegerin, RANKL, osteocalcin, and high-sensitivity CRP (hs-CRP) along with urine concentration of beta (c) cross labs were measured. Serum level of osteocalcin and osteoprotegerin were measured by N-MID®Osteocalcin enzyme-linked immunosorbent assay (ELISA; Immuno Diagnostic Systems, Tyne & Wear, UK) and osteoprotegerin Human ELISA Kit (ab100617) respectively. Urine level of β-cross laps was measured by Urine β-CrossLaps®ELISA (Immuno Diagnostic Systems). Serum concentration of hs-CRP and RANKL were assessed by CRP Human ELISA Kit (ab99995) and Human RANKL ELISA Kit from DLdevelop respectively.
The CACS was calculated using the method of Agatston et al.[8] by an experienced radiologist.
The FRAX score which was developed by World Health Organization (WHO) provides risk estimation for probability of fracture in the next 10 years. The score is calculating based on clinical risk factors and bone mineral density (BMD) at the femoral neck. This model of risk assessment has been developed by population studies for European, North American, Asian countries and Australia.[9] Because there is no FRAX tool for Iranians, we used computer driven FRAX tool for Lebanese who seems to be more similar with Iranians.
One-sample Kolmogorov-Smirnov test was used to assess normal distribution of the data. All data were normally distributed and no transformation was made (P>0.05). To recognize the association between CACS and BMD, bone biomarkers and FRAX score, multiple linear regression model was used. Several covariates for adjustment were chosen including age, weight, body mass index (BMI), and years since menopause.
All statistical analyses were performed with SPSS 18.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as mean means±standard deviations (SD). All P-values of less than 0.05 were considered to indicate statistical significance.
Eleven postmenopausal women who had CACS higher than 80 were enrolled in the study. The demographic data and past medical history of all study participants are summarized in Table 1.
The mean CACS was 563.82 (484.39). The mean serum concentration of osteocalcin was 17.15±8.22. The association between ca score and serum osteocalcin concentration was significant (std β=0.66, 95% confidence interval [CI]=5.47-72.27, P=0.02). After adjustment for age, weight, BMI, and years since menopause the association remained significant (std β=0.89, 95% CI=29.92-82.24, P=0.01).
The mean femoral BMD of all participants was 0.69±0.085. Our analysis showed significant association between ca score and femoral BMD (std β=−0.6, 95% CI=−6,864.34-14.27, P=0.05) which remained significant after adjustment for age, weight, BMI, and years since menopause (std β=−1.3, 95% CI=−12,825.84-2,249.83, P=0.01).
Participants' femoral T-score was -1.45±0.75. Significant association between CACS and femoral T-score was found (std β=−0.6, 95% CI=−773.08-1.28, P=0.05) which remained significant after adjustment for age, weight, BMI, and years since menopause (std β=−1.11, 95% CI=−1,379.91-49.78, P=0.04).
The mean serum concentration of osteoprotegerin in all participants was 4.74±0.67. No significant association was found between osteoprotegerin and calcium score (std β=−0.05, 95% CI=−583.03-503.42, P=0.87).
The mean urine concentration of β cross laps was 0.51±0.36. No significant association was detected between β cross laps and CACS (std β=0.29, 95% CI=−556.12-1,348.97, P=0.37). After adjustment for age, weight, BMI, and years since menopause the association found to be significant (std β=0.92, 95% CI=20.42-2,439.42, P=0.04).
The mean patients' FRAX score (the ten year probability of fracture %) for major osteoporotic fracture was 7.79 (3.21) and for hip fracture was 2.54±3.24. No association between patients FRAX score and CACS was observed.
No more correlation was found between study variables. Serum concentration of RANKL and hs-CRP was undetectable except for one participant. Therefore we omitted those data from analysis.
This study conducted on postmenopausal women who had CACS higher than 80 and were considered to be at high risk of atherosclerosis. Our results showed that CACS is independently associated with osteocalcin. Osteoclacin is a bone formation marker which originates from skeleton and indicates the osteoblasts potential for making bone matrix. It is a marker of bone mineralization and calcium homeostasis[1] and recently was recognized as a strong predictor of severity of coronary atherosclerosis.[10]
In agreement with our study Kim et al.[11] observed significant positive correlation between aortic CACS and osteocalcin, vertebral fractures and total hip-trabecular BMD (tBMD). A recent cohort study in Chinese men indicated serum osteocalcin as a strong determinant of severity of CAD.[10] In contrast Awan et al.[12] reported a negative association between vascular calcification and serum osteocalcin in familiar hypercholesterolemia and also Wilund et al.[13] found an inverse significant correlation between logarithmic value of CACS and plasma osteocalcin.
Chen et al.[14] observed significant lower levels of serum osteocalcin in patients with self-reported CVD in comparison with middle-aged and elderly Chinese without CVD. In addition Sheng et al.[15] observed that reduced osteocalcin is associated with increased risk of carotid atherosclerosis plaques in type 2 diabetes patients. They indicated decreased serum concentration of osteocalcin as a high risk for carotid atherosclerotic plaques and presented osteocalcin as a promising candidate for risk assessment of CVD. We included patients with CACS more than 80 which may explain the reason of the difference between our results with those who reported a converse relationship between CACS and osteocalcin. It was suggested that osteocalcin may have preventive effect on arteriosclerosis[16] while our patients CACS was high enough to show atherosclerosis. So there is the possibility that the severity of atherosclerosis (based on CACS) determines serum osteocalcin level.
Although the mean serum concentration of osteoprotegerin in our study was higher than normal, no association was found between CACS and osteoprotegerin serum concentration even after adjustment for age, weight, BMI and years since menopause. It was observed that the elevated level of osteoprotegerin is associated with severity of CAD.[1] It has been reported that osteoprotegerin is higher in patients with 2 to 3 vessels atherosclerosis while its level in patients with 1 vessel atherosclerosis is similar to those without CAD.[17] The positive correlation of the level of osteoprotegerin and CAC was demonstrated in diabetic patients whose mean serum osteoprotegerin concentration was higher than our patients.[18] Asanuma et al.[19] observed an association between osteoprotegerin and CACS in patients with rheumatoid arthritis. Also there are reports on the effect of angiotensin receptor blockers on reducing osteoprotegerin secretion from carotid endarterectomy samples taken from stroke patients[20] which may more explain our results. Furthermore changes in the local vascular expression of osteoprotegerin may not be directly proportional to its circulating level.[21]
Bakhireva et al.[22] reported higher osteoprotegerin levels in women with CAC compared with no/minimal calcification which was disappeared after age adjustment. They found significant lower odds of having CAC with 1 SD increase in hip BMD. Other clinical studies also demonstrated the direct association between osteoprotegerin level and severity of CAD, vascular dysfunction and cardiovascular mortality.[23] According to the counteraction between osteoprotegerin and RANKL, we measured serum RANKL in our patients but unfortunately we could not detect its level. Therefore the interaction between osteoprotegerin and RANKL in association with CACS should be evaluated in future larger studies.
Our results showed an inverse association between CACS and BMD and T-score at femur (but not spine) which remained significant after adjustment for age, weight, BMI and years since menopause. This is in line with other previous studies which confirm this result with bone density at spine,[24252627] however to date no study evaluated T-score. Choi et al.[28] observed an inverse correlation between CACS and BMD at femur which was stronger in women with a longer time since menopause and women with osteoporosis and osteopenia than normal BMD. Some researchers have indicated the site specific bone densitometry. In contrast to our study the association of lower spine volumetric BMD (vBMD) with high aortic calcification but not to CAC was reported in a cross sectional study,[29] however the CACS of our study participants was higher than those. In middle-aged postmenopausal women with extensive coronary plaque burden Lin et al.[30] did not find an association between coronary calcification and low BMD. In addition the association between vertebral bone density and CACS was determined by Filgueira et al.[31] in non-dialyzed chronic kidney disease (CKD) patients. Accordingly Carr et al.[32] demonstrated an association between vertebral trabecular bone density and vascular calcification. They explained their finding by introducing vertebral trabecular bone as the active metabolic bone which its density provides a measurement of volumetric BMD. In agreement with our study bone densitometry at spinal sites failed to show association with vascular calcification.[33]
Bakhireva et al.[34] reported no age-independent association between BMD at any site and CACS in men and women not using hormone therapy (HT) while the association was significant in HT users and they suggested that estrogen may mediated the association. Ramsey-Goldman and Manzi [35] observed an inverse significant correlation between CACS and lumbar spine BMD and hip BMD in a pilot study in young women with Lupus.
Based on the importance of the serum concentration of hs-CRP in atherosclerosis and CVDs and former reports indicating higher hs-CRP levels in osteopenic and osteoporotic patients[36] we proposed to measure its level in our patients and its association with other markers and scores, but we could not detect it in our patients.
We evaluated the association between CACS and FRAX which was not significant. To date this study was the first one provided FRAX and assessed its association with CACS which have to be precisely re-assessed in larger study.
Our results showed that after adjustment for age, weight, years since menopause and BMI the urine concentration of β cross laps have significant positive association with CACS. It may assumed that in patients with high CACS and high risk of CAD the rate of bone metabolism is high, so we observed the positive association between CACS and bone formation marker (osteocalcin) and bone resorption marker (β cross laps).
The study is a pilot observational study with limitations that has to be taken into account. The small sample size, not assessing patients with low CACS (lower than 80), and enrolling only patients with high CACS are limitations of this study. However, future larger studies with new methods of BMD and calcium scoring are necessitated. The study participants were chosen from patients referring for computed tomography-angiography therefore they are not representative of general population. The sample size is small enough to extrapolate the data to general population, but provides evidence for further extensive studies in larger sample size.
Taken together the association between CACS and osteocalcin, femoral BMD and T-score were strong enough to be observed, however, the potential associations between RANKL/osteoprotegerin system and CAC needs to be clarified in larger studies because finding the way of antagonizing this pathway may benefit both cardiovascular system and bone. The underlying mechanisms of the above mentioned associations are still under question. Although we considered the most potential contributors, still the possible effect of the other underlying diseases and medications is not completely understood. Therefore further population based studies are highly recommended.
ACKNOWLEDGEMENT
The study was approved and financially supported by Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences. The authors would like to thank Dr. Farideh Razi, Miss. Sara Shirazi, Dr. Patrishia Khashayar, Dr. Neda Mehrdad and Mrs. Espandbod for their great help and assistance. Also we thank all patients' participation.
References
1. Salari P, Abdollahi M. A comprehensive review of the shared roles of inflammatory cytokines in osteoporosis and cardiovascular diseases as two common old people problem: actions toward development of new drugs. Int J Pharmacol. 2011; 7:552–567.

2. Nadra I, Mason JC, Philippidis P, et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res. 2005; 96:1248–1256. PMID: 15905460.
3. Min H, Morony S, Sarosi I, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000; 192:463–474. PMID: 10952716.

5. Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001; 21:1998–2003. PMID: 11742876.

6. Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography). Circulation. 2007; 115:402–426. PMID: 17220398.
7. Krajnc M, Pečovnik-Balon B, Hojs R, et al. Comparison of coronary artery calcification and some coronary artery calcification risk factors in patients on haemodialysis and in patients with type 2 diabetes. J Int Med Res. 2011; 39:1006–1015. PMID: 21819735.

8. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832. PMID: 2407762.

9. Kanis JA, McCloskey EV, Johansson H, et al. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010; 21(Suppl 2):S407–S413. PMID: 20464374.

10. Bao Y, Zhou M, Lu Z, et al. Serum levels of osteocalcin are inversely associated with the metabolic syndrome and the severity of coronary artery disease in Chinese men. Clin Endocrinol (Oxf). 2011; 75:196–201. PMID: 21521333.

11. Kim KJ, Kim KM, Park KH, et al. Aortic calcification and bone metabolism: the relationship between aortic calcification, BMD, vertebral fracture, 25-hydroxyvitamin D, and osteocalcin. Calcif Tissue Int. 2012; 91:370–378. PMID: 23052223.

12. Awan Z, Alwaili K, Alshahrani A, et al. Calcium homeostasis and skeletal integrity in individuals with familial hypercholesterolemia and aortic calcification. Clin Chem. 2010; 56:1599–1607. PMID: 20702785.

13. Wilund KR, Tomayko EJ, Evans EM, et al. Physical activity, coronary artery calcium, and bone mineral density in elderly men and women: a preliminary investigation. Metabolism. 2008; 57:584–591. PMID: 18328364.

14. Chen L, Li Q, Yang Z, et al. Osteocalcin, glucose metabolism, lipid profile and chronic low-grade inflammation in middle-aged and elderly Chinese. Diabet Med. 2013; 30:309–317. PMID: 22913521.

15. Sheng L, Cao W, Cha B, et al. Serum osteocalcin level and its association with carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2013; 12:22. PMID: 23342952.

16. Ogawa-Furuya N, Yamaguchi T, Yamamoto M, et al. Serum osteocalcin levels are inversely associated with abdominal aortic calcification in men with type 2 diabetes mellitus. Osteoporos Int. 2013; 24:2223–2230. PMID: 23563931.

17. Jono S, Ikari Y, Shioi A, et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002; 106:1192–1194. PMID: 12208791.

18. Ishiyama M, Suzuki E, Katsuda J, et al. Associations of coronary artery calcification and carotid intima-media thickness with plasma concentrations of vascular calcification inhibitors in type 2 diabetic patients. Diabetes Res Clin Pract. 2009; 85:189–196. PMID: 19497632.

19. Asanuma Y, Chung CP, Oeser A, et al. Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis. 2007; 195:e135–e141. PMID: 17570371.

20. Golledge J, McCann M, Mangan S, et al. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke. 2004; 35:1636–1641. PMID: 15143295.

21. Quercioli A, Luciano Viviani G, Dallegri F, et al. Receptor activator of nuclear factor kappa B ligand/osteoprotegerin pathway is a promising target to reduce atherosclerotic plaque calcification. Crit Pathw Cardiol. 2010; 9:227–230. PMID: 21119343.

22. Bakhireva LN, Laughlin GA, Bettencourt R, et al. Does osteoprotegerin or receptor activator of nuclear factor-kappaB ligand mediate the association between bone and coronary artery calcification? J Clin Endocrinol Metab. 2008; 93:2009–2012. PMID: 18319315.
23. Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004; 95:1046–1057. PMID: 15564564.

24. Barengolts EI, Berman M, Kukreja SC, et al. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998; 62:209–213. PMID: 9501953.

25. Hyder JA, Allison MA, Criqui MH, et al. Association between systemic calcified atherosclerosis and bone density. Calcif Tissue Int. 2007; 80:301–306. PMID: 17505774.

26. Celik C, Altunkan S, Yildirim MO, et al. Relationship between decreased bone mineral density and subclinical atherosclerosis in postmenopausal women. Climacteric. 2010; 13:254–258. PMID: 20082602.

27. Lee SH, Park SJ, Kim KN, et al. Coronary calcification Is reversely related with bone and hair calcium: the relationship among different calcium pools in body. J Bone Metab. 2016; 23:191–197. PMID: 27965940.

28. Choi SH, An JH, Lim S, et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin Endocrinol (Oxf). 2009; 71:644–651. PMID: 19226260.

29. Farhat GN, Cauley JA, Matthews KA, et al. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J Bone Miner Res. 2006; 21:1839–1846. PMID: 17002567.

30. Lin T, Liu JC, Chang LY, et al. Association between coronary artery calcification using low-dose MDCT coronary angiography and bone mineral density in middle-aged men and women. Osteoporos Int. 2011; 22:627–634. PMID: 20552331.

31. Filgueira A, Carvalho AB, Tomiyama C, et al. Is coronary artery calcification associated with vertebral bone density in nondialyzed chronic kidney disease patients? Clin J Am Soc Nephrol. 2011; 6:1456–1462. PMID: 21617086.

32. Carr JJ, Register TC, Hsu FC, et al. Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: the diabetes heart study. Bone. 2008; 42:43–52. PMID: 17964237.

33. van der Klift M, Pols HA, Hak AE, et al. Bone mineral density and the risk of peripheral arterial disease: the Rotterdam Study. Calcif Tissue Int. 2002; 70:443–449. PMID: 11976772.

34. Bakhireva LN, Barrett-Connor EL, Laughlin GA, et al. Differences in association of bone mineral density with coronary artery calcification in men and women: the Rancho Bernardo Study. Menopause. 2005; 12:691–698. PMID: 16278612.

35. Ramsey-Goldman R, Manzi S. Association of osteoporosis and cardiovascular disease in women with systemic lupus erythematosus. Arthritis Rheum. 2001; 44:2338–2341. PMID: 11665974.

36. Koh JM, Khang YH, Jung CH, et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005; 16:1263–1271. PMID: 15702263.

Table 1
Patients' characteristics
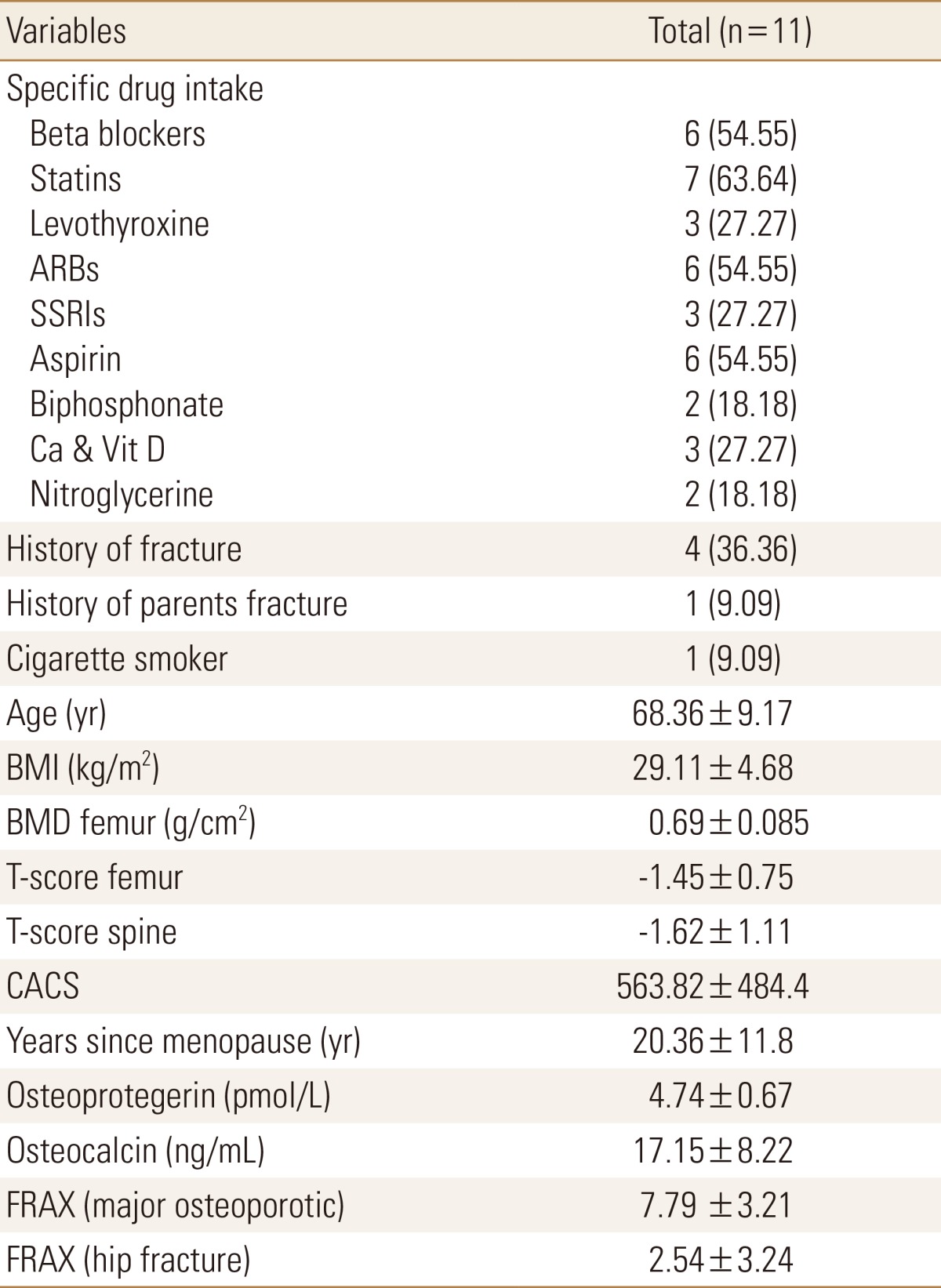
The data is presented as mean±standard deviation or number (%).
ARBs, angiotensin receptor blockers; SSRIs, selective serotonin reuptake inhibitors; Ca, calcium; CACS, coronary artery calcium score; Vit D, vitamin D; BMI, body mass index; BMD, bone mineral density; FRAX, fracture-risk assessment tool.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download