Abstract
Background
Lipocalin-2 (LCN2), a small glycoprotein, has a pivotal role in diverse biological processes such as cellular proliferation and differentiation. We previously reported that LCN2 is implicated in osteoclast formation induced by receptor activator of nuclear factor-kappa B ligand (RANKL) and macrophage colony-stimulating factor (M-CSF). In the present study, we used a knockout mouse model to further investigate the role of LCN2 in osteoclast development.
Methods
Osteoclastogenesis was assessed using primary bone marrow-derived macrophages. RANKL and M-CSF signaling was determined by immunoblotting, cell proliferation by bromodeoxyuridine (BrdU) enzyme-linked immunosorbent assay (ELISA), and apoptosis by cell death detection ELISA. Bone morphometric parameters were determined using a micro-computed tomography system.
Results
Our results showed that LCN2 deficiency increases tartrate-resistant acid phosphatase (TRAP)-positive multinucleated osteoclast formation in vitro, a finding that reflects enhanced proliferation and differentiation of osteoclast lineage cells. LCN2 deficiency promotes M-CSF-induced proliferation of bone marrow macrophages (BMMs), osteoclast precursors, without altering their survival. The accelerated proliferation of LCN2-deficient precursors is associated with enhanced expression and activation of the M-CSF receptor, c-Fms. Furthermore, LCN2 deficiency stimulates the induction of c-Fos and nuclear factor of activated T cells c1 (NFATc1), key transcription factors for osteoclastogenesis, and promotes RANKL-induced inhibitor of kappa B (IκBα) phosphorylation. Interestingly, LCN2 deficiency does not affect basal osteoclast formation in vivo, suggesting that LCN2 might play a role in the enhanced osteoclast development that occurs under some pathological conditions.
Osteoclasts are terminally differentiated multinucleated cells responsible for resorbing bone matrix and are also critical to the maintenance of bone remodeling and mineral homeostasis.[12] These cells are generated by the proliferation, fusion, and differentiation of hematopoietic precursors of the monocyte/macrophage lineage. Two molecules, receptor activator of nuclear factor-kappa B (NF-kB) ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) are essential and sufficient for osteoclast development.
M-CSF initiates cellular responses by binding to its receptor, c-Fms. Binding of M-CSF triggers receptor dimerization and tyrosine autophosphorylation, which, in turn, leads to the activation of downstream signaling pathways such as mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) and phosphatidylinositol (PI)3/Akt. These cellular events provide the signals necessary for proliferation and survival of osteoclast precursor cells. While the mitogen-activated protein kinase (MAPK) pathway, MEK/ERK, primarily supports proliferation of macrophages (osteoclast precursors),[345] PI3K/Akt mediates survival,[6] proliferation,[7] and cytoskeletal organization.[8]
Interaction of RANKL with its receptor, RANK, stimulates the induction and activation of several transcription factors including NF-κB, c-Fos, and nuclear factor of activated T cells c1 (NFATc1), all of which are crucial for osteoclast differentiation. NFATc1 can then induce the expression of osteoclastogenic genes such as tartrate-resistant acid phosphatase (TRAP) and cathepsin K in cooperation with other transcription factors including AP-1 and PU.1.[9]
Lipocalin-2 (LCN2), also known as neutrophil gelatinase-associated lipocalin (NGAL), is a small glycoprotein belonging to the lipocalin family, which functions as a carrier, transporting lipids and other hydrophobic molecules under physiological conditions. LCN2 was initially reported to exert antibacterial activity by chelating bacterial siderophores.[1011] Consequently, LCN2-deficient mice are highly sensitive to bacterial infection.[12] Moreover, LCN2 plays an important role in various cellular processes, such as cellular survival,[131415] proliferation,[10111617] and differentiation.[1819]
We recently demonstrated that LCN2 and its receptors, megalin and 24p3 receptor (24p3R), are expressed in osteoclast lineage cells.[20] Additionally, we demonstrated that ectopic expression or treatment of recombinant LCN2 suppresses proliferation and differentiation of osteoclast lineage cells, thus inhibiting osteoclast formation.[20] In the present study, we further investigated the role of LCN2 in osteoclast development mediated by M-CSF and RANKL, using a LCN2-deficient mouse model (LCN2-/- mice). Results showed that LCN2 deficiency enhances macrophage proliferation and osteoclast differentiation in vitro; events that are related to increased c-Fms expression and RANKL-induced NF-κB activation. Hence, these observations led us to examine bone phenotype of LCN2-/- mice, to determine the role of LCN2 in vivo.
LCN2-/- mice were provided by Dr. Shizou Akira (Osaka University, Japan) and were backcrossed with C57BL/6 wild-type (WT) mice for more than six generations.[21] Heterogenic mice were mated, and littermates used for experiments. All animal studies were conducted following the guidelines established by the Committee on the Ethics of Animal Experiments of the Kyungpook National University. Bone morphometric parameters and micro-architectural properties of the femur were determined using a micro-computed tomography (µCT) system (Quantum FX micro-CT; PerkinElmer, Waltham, MA, USA). Analysis of bone histology was performed as previously described.[22]
Osteoclasts were derived from mouse bone marrow cells as previously described.[20] Briefly, whole bone marrow cells were cultured in alpha-minimal essential medium (MEM) containing 10% fetal bovine serum (FBS) with M-CSF for 3 days. The adherent cells (bone marrow macrophages [BMMs]) were used as osteoclast precursors. To differentiate cells into osteoclasts, BMMs were cultured with M-CSF (10 ng/mL) and RANKL (20 ng/mL) for 4 days.
Cells were fixed in 4% paraformaldehyde and subsequently stained for TRAP activity with a 0.1 M acetate solution (pH 5.0) containing 6.76 mM sodium tartrate, 0.1 mg/mL naphthol AS-MX phosphate, and 0.5 mg/mL Fast Red Violet. Multinucleated cells positive to TRAP expression were identified as osteoclasts.
BMMs were cultured with various concentrations of M-CSF for 3 days. Subsequently, bromodeoxyuridine (BrdU) was added to the culture medium and incubated for 4 hr. Proliferation assay was performed using a cell-proliferation Biotrak ELISA system (GE Healthcare/Amersham, Freiburg, Germany). Apoptosis assay was conducted using the Cell Death Detection ELISA Kit (Roche Diagnostics, Mannheim, Germany), to detect cytoplasmic histone-associated DNA fragmentation.
Real-time quantitative PCR was performed using the SYBR Green dye in the Applied Biosystem (ABI) 7500 real-time PCR machine (Applied Biosystems, Foster City, CA, USA). Primers were designed using Primer Express software (Applied Biosystems). The amplification reaction was performed for 40 cycles with denaturation at 95℃ for 10 min followed by annealing at 95℃ for 15 sec and extension and detection at 60℃ for 1 min. The following primers were used: c-Fos, 5'-AGGCCCAGTGGCTCAGAGA-3' and 5'-GCTCCCAGTCTGCTGCATAGA-3'; NFATc1, 5'-ACCACCTTTCCGCAACCA-3' and 5'-TTCCGTTTCCCGTTGCA-3'; TRAP, 5'-TCCCCAATGCCCCATTC-3' and 5'-CGGTTCTGGCGATCTCTTTG-3'; and LCN2, 5'-CACAGGTATCCTCAGGTACAGAGCTA-3' and 5'-GGAAAAATACCATGGCGAACTG-3'.
Cells were harvested after washing with ice-cold phosphate buffered saline (PBS) and then lysed in radioimmunoprecipitation assay (RIPA) buffer containing 50 mM Tris (pH 7.4), 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 1 µg/mL pepstatin, and 1 µg/mL aprotinin. Whole cell lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was probed with specific antibodies, and immuno-reactivity was detected using enhanced chemiluminescence reagents (ECL Plus; Amersham Biosciences, Piscataway, NJ, USA). Antibodies against phospho-c-Fms, phospho-inhibitor of kappa B (IκBα), phospho-c-Jun, phospho-p38, phospho-ERK, phospho-Akt, p38, ERK, and Akt were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies for c-Fms and c-Fos were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibody for NFATc1 was purchased from BD Pharmingen (San Diego, CA, USA).
Our previous study demonstrated that LCN2 regulates osteoclast formation.[20] In the present study, we used LCN2-deficient (LCN2-/-) mice, to further investigate the role of LCN2 in osteoclast development. BMMs derived from WT and LCN2-/- mice were cultured for 4 days in osteoclastogenic media containing M-CSF and two different concentrations of RANKL. As expected, RANKL treatment of WT BMMs increased the number of TRAP-positive multinucleated cells in a dose-dependent manner (Fig. 1A, B). Compared with WT counterparts, LCN2 deficiency resulted in a significantly increase of osteoclast numbers in both RANKL concentrations (Fig. 1A, B).
The rate of precursor proliferation and apoptosis are important factors in determining osteoclast numbers. Therefore, increased osteoclast formation, due to LCN2 deficiency, could be caused by greater proliferation of osteoclast precursors and/or decreased apoptosis. To investigate whether LCN2 deficiency affects these parameters, we first assessed the apoptotic rate of WT and LCN2-/- BMMs by a DNA fragmentation assay with ELISA. We found that apoptotic rates are comparable in both WT and LCN2-/- cells (Fig. 2A), demonstrating that accelerated osteoclast formation is not caused by the decreased cell death seen in LCN2-/- BMMs. Next, we examined the impact of LCN2 deficiency on the proliferation of osteoclast precursors. For this purpose, we cultured WT and LCN2-/- BMMs for 3 days in the presence of the various concentrations of M-CSF. As previously reported, M-CSF increased cell growth in a dose-dependent manner (Fig. 2B). In LCN2-/- BMMs, BrdU incorporation was markedly increased at all concentrations of M-CSF (Fig. 2B). Thus, LCN2 deficiency affects proliferation of osteoclast precursors, but not their survival, thereby resulting in enhanced osteoclast formation.
M-CSF promotes the proliferation of osteoclast precursor cells by binding to c-Fms, its unique tyrosine kinase receptor. Since c-Fms plays a critical role in BMM proliferation, we investigated whether LCN2 deficiency influences c-Fms expression. As shown in Figure 2C, expression of c-Fms was markedly increased in LCN2-/- BMMs, when compared to WT cells. Accordingly, the phosphorylation of c-Fms in response to M-CSF was enhanced in LCN2-/- BMMs. Binding of M-CSF to c-Fms promotes the activation of downstream signaling pathways. Hence, we assessed M-CSF-stimulated MAPK and PI3K/Akt activation and established that ERK phosphorylation was significantly increased in LCN2-/- BMMs (Fig. 2C). On the other hand, phosphorylation of Akt was only modestly increased in LCN2-/- BMMs.
Based on our observations, LCN2 deficiency increases osteoclast formation. Therefore, we examined whether LCN2 deficiency influences the RANKL-induced expression of c-Fos, a pivotal transcription factor for osteoclast differentiation. LCN2 deficiency accelerated the RANKL-mediated induction of c-Fos mRNA, when compared to WT control (Fig. 3A). In concordance with the mRNA increase, LCN2 deficiency enhanced RANKL-induced c-Fos protein levels (Fig. 3B). Since NFATc1 acts as a downstream target of c-Fos, we then examined the impact of LCN2 deficiency on the RANKL-dependent induction of NFATc1, another transcription factor essential for osteoclastogenesis. As shown in Figure 3, LCN2 deficiency increased both mRNA and protein expression of NFATc1 in response to RANKL. Mirroring the increased expression levels of NFATc1, expression of osteoclastogenic markers such as TRAP and cathepsin K, was also enhanced as a result of LCN2 deficiency (Fig. 3A, B). We also observed that LCN2 receptors, 24p3R and megalin were expressed in both WT and LCN2-/- cells (Fig. 3C).
RANKL controls osteoclastogenesis through the activation of downstream signaling pathways such as NF-κB and MAPKs. To determine the mechanism by which LCN2 regulates osteoclast differentiation, we examined the impact of LCN2 deficiency on RANKL signaling cascades. BMMs from WT and LCN2-/- mice were serum-starved and exposed to RANKL, to then perform an immunoblotting assay. While phosphorylation of p38 MAPK was not affected by LCN2 deficiency, we observed a marked enhancement of IκBα and c-Jun phosphorylation in LCN2-/- BMMs following RANKL stimulation (Fig. 3D).
To investigate the role of LCN2 in vivo, we characterized the bone phenotype of LCN2-/- mice. Interestingly, micro-computed tomography analysis of the distal femur from 2-month-old LCN2-/- and WT mice showed no significant differences between strains (Fig. 4A). Both WT and LCN2-/- mice had similar bone volume per tissue volume (BV/TV), bone mineral density (BMD), trabecular number (Tb. N), and trabecular space (Tb. Sp) (Fig. 4B). Histological examination also revealed no apparent differences between WT and LCN2-/- mice (Fig. 4C). Similarly, serum levels of C-terminal telopeptides of type I collagen (CTX-1) were unaltered; suggesting that in vivo basal osteoclast development in LCN2-/- mice is normal (Fig. 4B). Together, these results suggest that LCN2 deficiency does not significantly impact osteoclast formation in vivo under normal physiological conditions.
We previously demonstrated that LCN2 is expressed in osteoclast lineage cells and that its expression is regulated during osteoclastogenesis. Subsequent studies, including ectopic expression and recombinant LCN2 protein treatment, revealed that LCN2 suppresses the proliferation and differentiation of osteoclast precursors. In the present study, we used a genetically engineered LCN2-/- mice and their WT littermates, to confirm that LCN2 negatively regulates in vitro osteoclast formation in response to M-CSF and RANKL.
It has been shown that LCN2 plays a critical role in cell proliferation. LCN2 inhibits the growth of bacteria through the formation of a complex with bacterial siderophore.[10, 11] Furthermore, a previous study using LCN2-/- mice, revealed that LCN2 suppresses bacterial growth, thereby protecting host against bacterial infection.[12] In the context of hematopoiesis, LCN2 also has been reported to suppress the growth of erythroid and monocyte/macrophage progenitors, in both mice and humans.[1617] Consistent with these findings and results from our previous work,[20] LCN2 deficiency accelerates the proliferation of osteoclast precursor cells, BMMs. On the other hand, LCN2 deficiency did not influence the apoptosis of osteoclast progenitors.
Osteoclast number is determined by proliferation, differentiation, and apoptosis rates of osteoclast lineage cells. The terminally differentiated osteoclasts are non-proliferative, but their precursors can proliferate under the control of M-CSF and its receptor c-Fms. The importance of M-CSF in osteoclast biology has been well demonstrated in previous studies. The naturally occurring op/op mice, which cannot express functional M-CSF due to a mutation in the csf1 gene, develop osteopetrosis due to complete absence of macrophages and osteoclasts.[2324] The critical role of c-Fms in osteoclast development was demonstrated using genetically modified mice. Mice-deficient csf1r, the gene coding for c-Fms, exhibit the same major phenotype as the op/op mice as a result of a significant decrease in macrophages and lack of mature osteoclasts.[25] In the present study, we observed that LCN2 deficiency enhances the expression and activation of c-Fms. Thus, accelerated proliferation of LCN2-/- BMMs is consistent with the increased expression of c-Fms protein. Consequently, downstream signaling pathways such as ERK and Akt, were increased due to LCN2 deficiency. Collectively, studies show that LCN2 suppresses M-CSF-induced precursor proliferation through the regulation of c-Fms expression, which in turn results in defective osteoclast formation.
c-Fos and NFATc1 are key modulators of RANKL-induced osteoclastogenesis.[92627] Our previous work demonstrates that overexpression or treatment with recombinant LCN2 attenuates RANKL-induced c-Fos and NFATc1 expression. Consistent with our previous results, we observed that c-Fos and NFATc1 induction was significantly enhanced in BMMs and osteoclasts derived from LCN2-/- mice. Consequently, NFATc1 leads to the increased expression of TRAP and cathepsin K, which are required for osteoclast differentiation and function.
Stimulation of RANKL activates MAPK signaling pathways including c-Jun N-terminal kinase (JNK), ERK, and p38. These MAPKs signaling pathways then activate AP-1 components including c-Fos and c-Jun, which in turn enhance osteoclast differentiation. NF-κB activation by RANKL is another pivotal event for osteoclastogenesis. Consistent with accelerated osteoclast differentiation, phosphorylation of IκBα and c-Jun was increased in LCN2-/- BMMs. On the other hand, activation of p38 was not affected by LCN2 deficiency. Notably, our previous work revealed that addition of LCN2 inhibits IκBα phosphorylation, nuclear translocation of the NF-κB subunit p65, and NF-κB transcriptional activity in response to RANKL. Thus, suppression of RANKL-mediated NF-κB activation is likely to be an important component of anti-osteolastogenic properties of LCN2.
Our study provides clear evidence that LCN2 deficiency increases osteoclast formation in vitro. However, in contrast to in vitro data, in vivo basal bone phenotype is normal in LCN2-/- mice. One possible explanation is that osteoclast formation, in vitro, reflects an activated state. Likewise, mice lacking Lyn,[28] Fyn,[29] and NF-κB-inducing kinase (NIK),[30] also show no basal phenotype in vivo, despite the profound impact on osteoclast number in vitro. As these mice represent in vivo phenotype in an activated state, but not in a basal state, we speculate that LCN2 may also play a role in the accelerated osteoclast development that occurs under a variety of pathological conditions, including osteoporosis and rheumatoid arthritis. However, whether this is the case in the context of LCN2 needs to be further investigated. Nonetheless, a recent study demonstrated that LCN2-/- mice develop severe serum-induced arthritis when compared to WT mice.[31] The study demonstrated, through histological examination, that mice paws of LCN2-/- mice have significant bone erosion compared to their WT counterparts.[31] These studies indicate that LCN2 has a protective role in the development of inflammatory diseases and in the preservation of bone homeostasis. In this regard, our study provides another piece of evidence that LCN2 functions as a negative regulator of osteoclast development, when induced by M-CSF and RANKL.
Figures and Tables
Fig. 1
Lipocalin-2 (LCN2) deficiency increases osteoclast formation. Wild-type (WT) and LCN2-/- bone marrow macrophages were cultured with macrophage colony-stimulating factor (10 ng/mL) and the indicated concentrations of receptor activator of nuclear factor-kappa B ligand. (A) After 4 days, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP). (B) Statistical analysis of WT and LCN2-/- TRAP-positive multinucleated cells/well at day 4. Data are expressed as the mean standard deviation. *P<0.05. WT, wild-type; LCN2, lipocalin-2; RANKL, receptor activator of nuclear factor-kappa B ligand; TRAP, tartrate-resistant acid phosphatase; MNC, more than three nuclie.
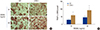
Fig. 2
Lipocalin-2 (LCN2) deficiency promotes precursor proliferation by enhancing expression and activation of c-Fms. (A) Wild-type (WT) and LCN2-/- bone marrow macrophages (BMMs) were cultured with macrophage colony-stimulating factor (M-CSF) (30 ng/mL). Apoptosis was determined as a function of DNA fragmentation. (B) WT and LCN2-/- BMMs were cultured with indicated concentrations of M-CSF. After 3 days, bromodeoxyuridine corporation was measured. Data are expressed as the mean standard deviation. *P<0.05, **P<0.001. (C) Serum-starved WT and LCN2-/- BMMs were stimulated with M-CSF (50 ng/mL) for the indicated time. Phosphorylated and total c-Fms were detected by immunoblotting; beta-actin served as a loading control. Phosphorylation of extracellular signal-regulated kinase (ERK) and Akt was determined by immunoblotting; total ERK and Akt levels served as loading controls. BrdU, bromodeoxyuridine; WT, wild-type; LCN2, lipocalin-2; M-CSF, macrophage colony-stimulating factor; ERK, extracellular signal-regulated kinase.

Fig. 3
Lipocalin-2 (LCN2) deficiency enhances receptor activator of nuclear factor-kappa B ligand (RANKL)-induced c-Fos expression and inhibitor of kappa B alpha phosphorylation. (A and B) wild-type (WT) and LCN2-/- bone marrow macrophages (BMMs) were cultured in osteoclastogenic media for the indicated days, and the expression of the indicated genes was analyzed by real-time polymerase chain reaction (PCR) (A) or immunoblotting (B). Data are expressed as the mean standard deviation. *P<0.05, ***P<0.001. (C) The expression of LCN2 receptors was determined by teal-time-PCR. (D) WT and LCN2-/- BMMs were treated with RANKL (50 ng/mL) for the indicated time. Immunoblots of total protein extracts were performed with the indicated antibodies; beta-actin and total p38 levels served as loading controls. WT, wild-type; LCN2, lipocalin-2; NFAT, nuclear factor-activated T cells; TRAP, tartrate-resistant acid phosphatase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RANKL, receptor activator of nuclear factor-kappa B ligand; IκBα, inhibitor of kappa B alpha.

Fig. 4
Bone phenotype of lipocalin-2 (LCN2)-/- mice. (A) Micro-computed tomography (µCT) images of distal femurs of wild-type (WT) and LCN2-/- mice. (B) µCT analysis of trabecular bone and the serum collagen type 1 fragment levels of WT and LCN2-/- mice. (C) Histological analysis of the proximal tibiae of WT and LCN2-/- mice; hematoxylin and eosin staining (top) and tartrate-resistant acid phosphatase staining (bottom). WT, wild-type; LCN2, lipocalin-2; TRAP, tartrate-resistant acid phosphatase; H&E, hematoxylin and eosin; BV, bone volume; TV, tissue volume; Tb.N, trabecular number; Tb.Sp, trabecular space; CTX-1, C-terminal telopeptides of type I collagen.

Notes
This study was supported by the Basic Science Research Program of the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science, and Technology (NRF-2015R1D1A1A01056666) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Korea (HI15C0001, HI14C2750).
References
1. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.

2. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003; 4:638–649.

3. Jaworowski A, Wilson NJ, Christy E, et al. Roles of the mitogen-activated protein kinase family in macrophage responses to colony stimulating factor-1 addition and withdrawal. J Biol Chem. 1999; 274:15127–15133.

4. Takeshita S, Faccio R, Chappel J, et al. c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J Biol Chem. 2007; 282:18980–18990.

5. Valledor AF, Comalada M, Xaus J, et al. The differential time-course of extracellular-regulated kinase activity correlates with the macrophage response toward proliferation or activation. J Biol Chem. 2000; 275:7403–7409.

6. Kelley TW, Graham MM, Doseff AI, et al. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999; 274:26393–26398.

7. Zhou P, Kitaura H, Teitelbaum SL, et al. SHIP1 negatively regulates proliferation of osteoclast precursors via Akt-dependent alterations in D-type cyclins and p27. J Immunol. 2006; 177:8777–8784.

8. Kim HJ, Zhao H, Kitaura H, et al. Glucocorticoids suppress bone formation via the osteoclast. J Clin Invest. 2006; 116:2152–2160.

9. Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901.

10. Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004; 432:917–921.

11. Goetz DH, Holmes MA, Borregaard N, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002; 10:1033–1043.

12. Berger T, Togawa A, Duncan GS, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006; 103:1834–1839.

13. Devireddy LR, Gazin C, Zhu X, et al. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005; 123:1293–1305.

14. Lee S, Lee J, Kim S, et al. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J Immunol. 2007; 179:3231–3241.

15. Nelson AM, Zhao W, Gilliland KL, et al. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest. 2008; 118:1468–1478.

16. Miharada K, Hiroyama T, Sudo K, et al. Lipocalin 2-mediated growth suppression is evident in human erythroid and monocyte/macrophage lineage cells. J Cell Physiol. 2008; 215:526–537.

17. Miharada K, Hiroyama T, Sudo K, et al. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005; 19:1881–1883.

18. Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008; 52:595–605.

19. Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002; 10:1045–1056.

20. Kim HJ, Yoon HJ, Yoon KA, et al. Lipocalin-2 inhibits osteoclast formation by suppressing the proliferation and differentiation of osteoclast lineage cells. Exp Cell Res. 2015; 334:301–309.

21. Jeon S, Jha MK, Ock J, et al. Role of lipocalin-2-chemokine axis in the development of neuropathic pain following peripheral nerve injury. J Biol Chem. 2013; 288:24116–24127.

22. Kim HJ, Yoon HJ, Choi JY, et al. The tyrosine kinase inhibitor GNF-2 suppresses osteoclast formation and activity. J Leukoc Biol. 2014; 95:337–345.

23. Kodama H, Nose M, Niida S, et al. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991; 173:1291–1294.

24. Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990; 87:4828–4832.

25. Dai XM, Ryan GR, Hapel AJ, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002; 99:111–120.

26. Ishida A, Fujita N, Kitazawa R, et al. Transforming growth factor-beta induces expression of receptor activator of NF-kappa B ligand in vascular endothelial cells derived from bone. J Biol Chem. 2002; 277:26217–26224.

27. Wang ZQ, Ovitt C, Grigoriadis AE, et al. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992; 360:741–745.

28. Kim HJ, Zhang K, Zhang L, et al. The Src family kinase, Lyn, suppresses osteoclastogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2009; 106:2325–2330.

29. Kim HJ, Warren JT, Kim SY, et al. Fyn promotes proliferation, differentiation, survival and function of osteoclast lineage cells. J Cell Biochem. 2010; 111:1107–1113.

30. Novack DV, Yin L, Hagen-Stapleton A, et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003; 198:771–781.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download