Abstract
Background
There are several studies about the relationship between serum homocysteine levels and bone mineral density (BMD), but the results are varied, and the studies are limited in Korea. In our study, the relationship between serum homocysteine levels and BMD by part according to age and sex is investigated.
Methods
From March 2012 to July 2015, the 3,337 healthy adults who took a medical examination were recruited. Subjects filled in the self-recording type questionnaire and physical examination, blood test, BMD of lumbar spine and femur were measured. After sorting by aging (≤49 year old, 50-59 year old, ≥60 year old) and sex, the results were adjusted with age and body mass index (BMI) and the relationship between serum homocysteine levels and BMD by lumbar spine and femur was analyzed by multiple regression analysis.
Results
As results of analysis, with the adjustment with age and BMI, all age groups of men had no significant relationship between log-converted serum homocysteine levels and BMD. In women aged under 50, there were significantly negative relationships at lumbar spine (β=-0.028, P=0.038), femur neck (β=-0.062, P=0.001), and total hip (β=-0.076, P<0.001), but there was no significant relationship in other age groups (50-59 year old and ≥60 year old).
Osteoporosis which increased risk of fracture is well known as a general common disease, and its complication can generate a social problem by lowering the quality of life and increasing the socioeconomic burden.[12] Aging, family history, malabsorption, underweight, and reduction in calcium intake are suggested as risk factors of osteoporosis, but the most important factor is the unacquired maximum bone mass in young age. If obtained bone mass is lower than standard, it can lead to osteoporosis at young age, and the age of osteoporosis occurrence become younger.[3] Men has lower prevalence rate in osteoporosis than women does, so the importance was overlooked relatively. But, due to the extension of the average life expectancy, the number of male osteoporosis become increasing. Also, frequency of osteoporotic fracture in men is increased, it has been reported that 30% of total hip fractures occur in men. It is known men has higher mortality rate from hip fracture than women.[45]
The studies to find risk factors for the early diagnosis or prevention of osteoporosis are actively ongoing.[6] From the several recent studies, it is known that osteoporosis and cardiovascular disease have a similar pathophysiological mechanism on their onset, which has been thought to be a distinct disease with different pathogenesis.[7] Among them, homocysteine is a metabolite of methionine, produced during protein metabolism, and can be expressed by malnutrition for several enzymes or vitamins which are related the metabolic process. It also affected by age, sex, smoking, or disease like cancer.[8910] Hyperhomocysteinemia is known as a significant risk factor which caused cardiovascular disease by leading abnormality of vascular tissue in variety mechanisms,[11] and hereditary hyperhomocysteinemia is known as an increasing factor for the incident of osteoporosis and fracture by osteoporosis.[1213] Some cellular experiments reported homocysteine promotes osteoclast differentiation and inhibits apoptosis.[141516] It is considered inhibition of collagen crosslink caused osteopenia, but the exact mechanism is not identified yet.[1718]
As the foreign related studies, the cohort study reported that people with higher serum homocysteine levels have higher risk of femoral fracture,[19] and some research reported increased homocysteine concentration which related to osteoporotic fractures is associated with bone mineral density (BMD) in the over 55 age group.[20] And a large scale cross-sectional studies reported there is negative relationship between the serum homocysteine levels and femoral BMD in the age group of 47 to 50 years and 71 to 75 years.[21] Some domestic study reported that there is no significant relationship between the serum homocysteine levels and BMD of lumbar spine or femur in postmenopausal woman,[22] and other study also reported homocysteine levels in middle-aged women cannot represent BMD and there is no significant effect on the bone metabolism.[23]
Although there are several studies on the relationship for serum homocysteine levels and BMD, the most studies targeted for the specific gender and specific ages. The domestic studies for including both sex and various ages are limited, and results have also been reported differently. Therefore, the aim of this study was to investigate the relationship between serum homocysteine levels and BMD by part according to age and sex.
From March 2012 to July 2015, 3,337 healthy adults who took a medical examination at the health promotion center in Jeju National University Hospital were recruited. Among them, the subjects with stroke, myocardial infraction, liver cirrhosis, renal failure, or other malignant disease were excluded, and serum homocysteine levels and BMD of 1,857 male and 1,480 female were collected.
Through the self-recording type questionnaire, medical history, smoking status, drinking status, and exercise status were investigated. We classified as the heavy alcohol consumption if average drinking was more than twice in a week and as a regular exercise if exercised more than three times a week. Physical examination was conducted with wearing a light examination gown only, and weight (kg), height (cm), waist circumference (measured at belly bottom in standing position), and body mass index (BMI; divide weight [kg] by the square of height [m]) were collected. To measure homocysteine levels, venous blood were collected in gastric emptying status (more than 8 hr) with chemiluminescent immunoassay using auto analyzer (Advia Centaur, Bayer Corp., Newbury, UK). BMD was measured by dual energy X-ray absorptiometry (DXA), and lumbar spine, femoral neck, and total hip were measured until 2 decimal points (g/cm2). Age was classified into under 50 years, between 50 to 59 years, and 60 or older.
The general characteristics of the age, height, weight, BMI, waist circumference, serum homocysteine levels, and BMD were noted as mean±standard deviation, and the status of smoking, drinking, and exercise were noted as number of people (percentile for total). Variance analysis was performed to determine the significant difference in BMD according to sex and age, and age and BMI were used as correcting factors during the covariance. The association between serum homocysteine levels and BMD were analyzed by sex and age with multiple linear regression analysis, and adjusted for age and BMII. SPSS for windows (Version 18.0; SPSS Inc., Chicago, IL, USA) were used for statistical analysis, and P-value for the significant level was less than 0.05 (P<0.05).
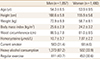
The variables of subject were as follows: 54.3±8.5 (age for men), 53.9±9.5 (age for women), 25.6±2.9 kg/m2 (BMI for men), 24.2±3.2 kg/m2 (BMI for women), 10.7±3.7 µmol/L (homocysteine levels for men), and 7.87±2.2 µmol/L (homocysteine levels for women) (Table 1).
BMI, separated by age in men, were 25.9±3.3 kg/m2 for age under 50, 25.7±2.7 kg/m2 for age between 50 and 59, and 25.1±2.9 kg/m2 for age 60 or older. BMI was lower in the older age group (P<0.001). Homocysteine levels were 11.0±4.6 µmol/L for age under 50, 10.3±3.0 µmol/L for age between 50 and 59, and 11.0±3.6 µmol/L for age 60 or older and there were statistically significant differences (P<0.001). There were also statistically significant differences in smoking, drinking, and exercise status (all P<0.001) (Table 2-1). In the case of women, when separated by age, BMI was higher in the older age group (P<0.001). Homocysteine levels were 7.3±1.9 µmol/L for age under 50, 7.7±1.9 µmol/L for age between 50 and 59, and 8.6±2.7 µmol/L for age 60 or older and the value were statistically significant difference (P<0.001) (Table 2-2).
Lumbar spine BMD was the lowest in 50 age group, and BMD of femur neck and total hip were lower in older age group (Unadjusted Pa)=0.014,<0.001,<0.001). After the adjustment for age and BMI, there was a difference in lumber spine, but there were no statistically significant differences in other part (Adjusted Pb)=0.008, 0.230, 0.649). The above results were presented in Table 3-1. In women's case, BMD for lumbar spine and total hip were lower in older age group. In the case of femur neck, 50 age group had the lowest BMD (Unadjusted Pa)<0.001,<0.001,<0.001). After the adjustment for age and BMI, the results of BMD for three groups were statistically significant differences (Adjusted Pb)<0.001, 0.002,<0.001) (Table 3-2).
Since the results for homocysteine don't show normal distribution, the log transformation value was used and age and BMI were adjusted. Since then, correlations with BMD were observed. In male age group between 50 and 59, BMD tended to decrease at femur neck and total hip, but the results were not statistically significant and there was no significantly correlation between BMD and serum homocysteine levels in other age groups (P>0.05) (Table 4-1). In the female age group under 50, there were significantly negative relationships at lumbar spine, femur neck and total hip (all P<0.05), but there were no significant correlations in other age groups (P>0.05) (Table 4-2).
For additional analysis, the ages for both genders were specifically classified into younger and older than 50 years. After the adjustment for age and BMI, the male age (more than 50) group's total hip BMD showed significantly negative relationship with log converted homocysteine (β=-0.028, P=0.022). In Female under 50 group, BMD at lumbar spine (β=-0.028, P=0.038), femur neck (β=-0.062, P=0.001), and total hip (β=-0.076, P<0.001) decreased significantly with increasing homocysteine.
In our study, we analyzed the relevance of serum homocysteine levels and BMD by adjusting age and BMI, and a significant negative correlation was observed between homocysteine and BMD of lumbar spine and femur in women aged under 50. These results had some matching point with other results of studies that there were negative correlations between serum homocysteine and BMD at lumbar spine and femur in 46 to 55 year old female and no association in male.[24]
The gender differences found in this study can be considered the possibility that the results came with less effect on homocysteine, because there are only few cases in very young age male have low BMD, and the interaction with the risk factors such as decreased gonadal function, alcohol consumption, and smoking [22] can be increased by increasing age. Also, excessive intake of animal protein that contains many methionine, smoking, or drinking can also lead to hyperhomocysteinemia,[2526] and generally the rate of animal protein intake is higher in men, and the number of smoking and drinking people that may affect to homocysteine levels are relatively higher to women's, so the impact by confounding factors such as smoking or drinking should be considered. The negative correlation in female under 50 which also has been reported in other studies was considered that homocysteine inhibited the collagen cross-linking during bone metabolism.[1718] Under normal physiological conditions, Increasing age gradually increased the blood levels of homocysteine, and the bone loss was relatively increased after 40 years old, so it caused significant decrease in BMD for postmenopausal women. After all, the idea that homocysteine increasing with aging and female hormone changes by menopausae affect the viscosity bone pathology could be considered.[2728] Besides, other reason that no statistically significant difference was observed in other age groups was the insufficient adjustment of nutritional status about vitamin cofactor such as folic acid, vitamin B12, or vitamin B6 which may affect the homocysteine metabolism.[29] Malnutrition is frequent by increasing age,[30] and it also associated with BMD beside the homocysteine.[3132] In addition, the possibility that the insufficient investigation for the currently taking medication or treatment for disease which may have correlation with BMD and serum homocysteine levels were also had effect to our results.
Regardless of previous studies which investigated correlations between homocysteine and BMD in menopausal women only, our study included young age and male objects. The study was more meaningful that show the requirement for BMD management and prevention of osteoporosis in young age through the maximum consumption of bone amount and the requirement of osteoporosis management for women as well as men.
Since the subjects in our study were visitor to the university hospital for a comprehensive examination, the representativeness was poor and the limitation of our study was unclear causal relationship from the cross-sectional study. Also, the investigation for factors that can give the effect to serum homocysteine levels such as animal protein intake habits, quantitative inspection for smoking and drinking, and current taking medication was insufficient. The investigation whether folic acid and vitamin intake which may affect to bone metabolism was not in progress. Beside the mentioned factors, the investigation to medication that affects BMD and blood homocysteine was insufficient.
Our cross-sectional study was research for the correlation between BMD and serum homocysteine levels which have been the interesting subject recently, and the investigation was targeting in Korea and based on the various age and gender which research was insufficient data meanwhile. Our study have significance that could reveal the correlation between BMD in various part and serum homocysteine levels. To support our study, future large-scale prospective cohort study about risk of bone fracture in various aspects are required by adjusting the other various factors that could affecting the serum homocysteine levels and the BMD.
Figures and Tables
Notes
References
1. Jeong TH, Kim CY, Sa KJ, et al. The effect of hormone replacement therapy and related factors on the change of bone mineral density in early postmenopausal women in Ulsan-si, Korea. J Korean Acad Fam Med. 2004; 25:233–243.
2. Cho S, Kim WS, Park JH, et al. The effect of hormone replacement therapy on bone mineral density in postmenopausal women. Korean J Obstet Gynecol. 1998; 41:475–482.
3. Gelfand IM, DiMeglio LA. Bone mineral accrual and low bone mass: a pediatric perspective. Rev Endocr Metab Disord. 2005; 6:281–289.

4. Eastell R, Boyle IT, Compston J, et al. Management of male osteoporosis: report of the UK consensus group. QJM. 1998; 91:71–92.

6. Lentle BC, Brown JP, Khan A, et al. Recognizing and reporting vertebral fractures: reducing the risk of future osteoporotic fractures. Can Assoc Radiol J. 2007; 58:27–36.
7. Burnett JR, Vasikaran SD. Cardiovascular disease and osteoporosis: is there a link between lipids and bone? Ann Clin Biochem. 2002; 39:203–210.

8. Brattström L, Israelsson B, Lindgärde F, et al. Higher total plasma homocysteine in vitamin B12 deficiency than in heterozygosity for homocystinuria due to cystathionine beta-synthase deficiency. Metabolism. 1988; 37:175–178.

9. Kang SS, Zhou J, Wong PW, et al. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988; 43:414–421.
10. Oh KW, Lee WY, Ahn YB, et al. Homocysteine, folate, and methylenetetrahydrofolate reductase polymorphism in Korean normal subjects. Korean J Med. 1999; 57:1030–1036.
11. Asfar S, Safar HA. Homocysteine levels and peripheral arterial occlusive disease: a prospective cohort study and review of the literature. J Cardiovasc Surg (Torino). 2007; 48:601–605.
13. Mudd SH, Skovby F, Levy HL, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985; 37:1–31.
14. Herrmann M, Widmann T, Colaianni G, et al. Increased osteoclast activity in the presence of increased homocysteine concentrations. Clin Chem. 2005; 51:2348–2353.

15. Koh JM, Lee YS, Kim YS, et al. Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. J Bone Miner Res. 2006; 21:1003–1011.

16. Kim DJ, Koh JM, Lee O, et al. Homocysteine enhances apoptosis in human bone marrow stromal cells. Bone. 2006; 39:582–590.

17. Lubec B, Fang-Kircher S, Lubec T, et al. Evidence for McKusick's hypothesis of deficient collagen cross-linking in patients with homocystinuria. Biochim Biophys Acta. 1996; 1315:159–162.

18. Whiting SJ, Draper HH. Effect of a chronic acid load as sulfate or sulfur amino acids on bone metabolism in adult rats. J Nutr. 1981; 111:1721–1726.

19. McLean RR, Jacques PF, Selhub J, et al. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med. 2004; 350:2042–2049.

20. van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004; 350:2033–2041.

21. Gjesdal CG, Vollset SE, Ueland PM, et al. Plasma total homocysteine level and bone mineral density: the Hordaland Homocysteine Study. Arch Intern Med. 2006; 166:88–94.

22. Bae SJ, Son HY, Kim DJ, et al. Lack of association between homocysteine levels and bone mineral density in healthy premenopausal and postmenopausal women. Korean J Bone Metab. 2004; 11:19–24.
23. Kim A, Lee JH, Lee JY, et al. Association between serum homocysteine concentrations and bone mineral density in middle aged women. J Korean Soc Menopause. 2013; 19:81–86.

24. Lee YJ, Lee SW, Lee HS, et al. The relationship of serum homocysteine levels with lumbar and femoral bone mineral density. Korean J Fam Med. 2008; 29:175–181.
25. Carmel R, Green R, Jacobsen DW, et al. Serum cobalamin, homocysteine, and methylmalonic acid concentrations in a multiethnic elderly population: ethnic and sex differences in cobalamin and metabolite abnormalities. Am J Clin Nutr. 1999; 70:904–910.

26. Rasmussen K, Møller J. Total homocysteine measurement in clinical practice. Ann Clin Biochem. 2000; 37(Pt 5):627–648.

27. Marcus R. Role of exercise in preventing and treating osteoporosis. Rheum Dis Clin North Am. 2001; 27:131–141. vi

28. Zhang J, Morgan SL, Saag KG. Osteopenia: debates and dilemmas. Curr Rheumatol Rep. 2013; 15:384.

30. Stone KL, Bauer DC, Sellmeyer D, et al. Low serum vitamin B-12 levels are associated with increased hip bone loss in older women: a prospective study. J Clin Endocrinol Metab. 2004; 89:1217–1221.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download