Abstract
Osteoporotic fractures are one of the most common causes of disability and a major contributor to medical care costs worldwide. Prior osteoporotic fracture at any site is one of the strongest risk factors for a new fracture, which occurs very soon after the first fracture. Bone mineral density (BMD) scan, a conventional diagnostic tool for osteoporosis, has clear limitations in diagnosing osteoporotic fractures and identifying the risk of subsequent fractures. Therefore, early and accurate diagnosis of osteoporotic fractures using the clinical definition which is applicable practically and independent of BMD, is essential for preventing subsequent fractures and reducing the socioeconomic burden of these fractures. Fractures caused by low-level trauma equivalent to a fall from a standing height or less at major (hip, spine, distal radius, and proximal humerus) or minor (pelvis, sacrum, ribs, distal femur and humerus, and ankle) sites in adults over age 50, should be first regarded as osteoporotic. In addition, if osteoporotic fractures are strongly suspected on history and physical examination even though there are no positive findings on conventional X-rays, more advanced imaging techniques such as computed tomography, bone scan, and magnetic resonance imaging are necessary as soon as possible.
The costs and implications of osteoporotic fractures for national health care systems are increasing rapidly, and as a result, intense efforts are being made to prevent second osteoporotic fractures in people who have already had first.[1] The World Health Organization (WHO) has defined osteoporosis as a metabolic bone disease characterized by low bone mass and microarchitectural deterioration of bone tissue leading to enhanced bone fragility and a consequent increase in fracture risk. The bone mineral density (BMD) scan is currently the gold standard assessment tool for diagnosing osteoporosis, which is measured at the lumbar spine and hip. However, diagnosing osteoporosis relying solely on BMD T-total scores identifies fewer than 50% of people who go on to have an osteoporotic fracture.[2] In addition, fractures at other sites such as the humerus or forearm contribute significantly to the burden of osteoporosis, particularly in younger individuals in whom osteoporotic fractures at sites other than the hip and spine are much more common.[3]
As populations age, a number of studies have classified fractures of the vertebrae, proximal femur, and distal radius as the main osteoporotic fractures and have also included fractures of the pelvis, subtrochanter and diaphysis of the femur, ankle, and rib.[234567] To date, the importance of fractures at sites other than the main fracture site has been emphasized as contributing to the numbers of fractures and increasing the socioeconomic burden.
As described above, osteoporotic fractures are defined as fractures at sites associated with low BMD, but low BMD alone might not fully detect the risk.[28] In addition, osteoporotic fractures are not always associated with low BMD. Therefore, more accessible and effective tool for diagnosing osteoporotic fractures is critical for reducing the risk and burden of subsequent fractures after the first one.
The objective of this review is to define osteoporotic fracture more practically and to present a more clinically applicable and useful tool for its diagnosis than the conventional method that depends only on areal BMD.
Since 1993, a number of studies regarding osteoporotic fracture have been conducted in Korea using cohort or nationwide medical claims database.[9101112] The first was by Rowe et al.,[11] who reported a hip fracture incidence of 33 per 100,000 adults (37/100,000 in men and 31/100,000 in women) using a cohort in Honam. These authors also performed a 10-year follow-up study in 2005 in Gwangju City and Chonnam Province and reported a hip fracture incidence of 133 per 100,000 adults (113/100,000 in men and 148/100,000 in women); the incidence increased fourfold over the 10-year study period.[10] Recently, a longitudinal cohort study of adults over age 50 on Jeju Island reported that the crude incidence of hip fractures had increased from 126.6 per 100,000 in 2002 (70.9/100,000 in men and 167.9/100,000 in women) to 183.7/100,000 in 2011 (89.4/100,000 in men and 261.9/100,000 in women).[9] The annual increase in hip fractures was 4.3% (5.3% in women and 2.2% in men).[8]
Members of the Korean Society for Bone and Mineral Research and the Health Insurance Review and Assessment Service (HIRA), using HIRA data, reported that the incidence of osteoporotic fractures (hip, spine, distal radius, and humerus), in adults aged 50 or over between 2005 and 2008 had increased from 189,856 in 2005 to 210,592 in 2008. In 2008, the incidence of spine fractures was highest (969 per 100,000 persons), followed by the distal radius (422), hip (157), and humerus (81).[12] These findings reflect a trend of increasing numbers of osteoporotic fractures in Korea.
Cortical porosity is relatively more marked in men, whereas cortical thinning prevails more in women, especially in the early stages.[13] The cortical bone, as a source of fragility, has more often been the focus for determining bone strength than the trabecular bone. Cortical bone loss occurs mainly at the endosteal surface and partly in the Haversian canals.[14] As endosteal resorption occurs, periosteal apposition also progresses to compensate, which partially preserves bone strength. However, cortical thinning reduces the resistance to compressive and bending forces, and is prone to leading to osteoporotic fracture.[15] Many studies have reported that the cortical thickness of bones including the tibia, humerus, metacarpal bone, and mandible can be used as an alternative for determining the risk of an osteoporotic fracture.[16171819] A recent review reported that the estimated cortical thickness of the mid-femoral neck might be of most importance in determining resistance to fracture.[20] Cortical porosity can be also used to identify the risk of osteoporotic fracture, but only the porosity of the outer compact-appearing cortex, not that of the inner transitional zone.[21]
Osteoporotic (fragility) fractures are fractures that result from mechanical forces that would not ordinarily result in a fracture, known as low-level (or low-energy) trauma according to National Institute for Health and Care Excellence (NICE) clinical guidelines. The WHO has quantified low-level trauma that causes osteoporotic fracture as force equivalent to a fall from a standing height or less. In addition, many clinicians consider the presence of an osteoporotic fracture (fracture caused by inadequate or mild trauma such as falling from standing height) as sufficient for a diagnosis of osteoporosis regardless of the patient's BMD.[22]
According to the conventional diagnosis based on the BMD T-score, osteoporosis is defined as T-score ≤-2.5 standard deviation (SD) or the presence of a prevalent fragility fracture despite T-score >-2.5 SD.[232425] Osteoporotic fractures are associated with low BMD measured at the fracture site. However, the occurrence of osteoporotic fracture is not always associated with low bone density equivalent to osteoporosis, and in most cases, central BMD measures assessed mainly at the lumbar spine and the proximal femur are used (Fig. 1).[26]
Based on the description above, clinical criteria are needed for defining osteoporotic fractures at sites other than the lumbar spine and proximal femur, which are not commonly used for measuring BMD, in order to recognize these fractures and initiate timely and appropriate therapy. The above-described definition of osteoporotic fracture can be easily obtained from the history of injury and radiographic findings. This clinically applicable definition of osteoporotic fracture regardless of BMD can determine the risk of second fractures and reduce the associated socioeconomic burdens.
Common osteoporotic fracture sites include bones that are under strain because they bear weight (such as the spine, hip, and pelvis) or that take the stress when a person catches him- or herself when falling from a standing height or less (such as the wrist, forearm, and upper arm).
Osteoporotic fractures occur mainly at sites that are associated with low BMD and increase in incidence after the age of 50.[27] Conventionally, the spine, hip, and distal radius have long been regarded as the quintessential osteoporotic fracture sites. However, large studies have shown that nearly all types of fractures occur more often in patients with low bone density irrespective of the site.[32829]
Patients who have fractures at typical osteoporotic sites (spine, hip, wrist, and humerus) are most likely to have low BMD, but 74% of patients with fractures at less typical sites (ankle, hand, foot, other sites) also have low BMD at either the hip or the spine.[30] This finding reinforces the recommendation that history of any low-trauma fracture at any site should be an indication for osteoporosis evaluation.
According to NICE and National Osteoporosis Foundation (NOF) guidelines, osteoporotic fractures occur most commonly in the spine, hip, and distal radius but may also occur in the humerus, pelvis, ribs, and other bones. The WHO considers proximal humerus fractures to be one of the major osteoporotic fractures.
Recently, fractures of the pelvic ring in older populations have been classified as osteoporotic because this fracture type is caused by low-energy trauma or no trauma in populations with osteoporosis. Low-energy falls are responsible for the majority of pelvic insufficiency fractures, and moreover, up to two-thirds of sacral insufficiency fractures have been noted to occur in the absence of trauma in older populations.[31] In addition, rib fractures from low-energy trauma have been reported as common non-vertebral fractures in the elderly.[3233] These studies revealed an increasing pattern of fracture incidence, and a history of rib fracture carried at least a twofold increased risk of a subsequent osteoporotic fracture. In addition, ankle fractures have gained increasing attention as another type of fragility fracture.[434] Low-energy ankle fractures can offer significant implications for identifying patients who need osteoporosis treatment. One population-based study identified radiologic findings and trauma history as valid tools for assessing osteoporotic ankle fractures.[35]
Following the description above, Table 1 lists what are considered to be the major and minor sites of osteoporotic fractures (Fig. 2, 3).
All of these fractures should be assessed based on the clinical and research evidence and considering the benefits of osteoporosis management, including reducing the risk of osteoporotic fractures.
Osteoporotic fractures and the associated costs are expected to increase markedly as populations age.[36] These fractures are associated with low bone mass, and their incidence increases with age after the age of 50. The estimated lifetime risk for an osteoporotic fracture among 50-year-old women in North America is approximately 18% for hip fracture, 16% for clinically diagnosed vertebral fracture, and 16% for distal radius fracture.[37] Overall, the NOF estimates that 1 in 2 white women and 1 in 4 white men older than age 50 will sustain at least 1 osteoporosis-related fracture in their remaining lifetimes.[38] Prior osteoporotic fracture in this population is an important predictor of future fractures.[39] Therefore, fractures at the sites described above in adults over age 50 should be strongly suspected as osteoporotic. Their neglect ultimately can underestimate the burden of osteoporosis and lead to subsequent fractures, particularly in relatively younger individuals.
Finally, a recent fracture at any skeletal site described above in an adult older than 50 years of age should be considered a significant event for the diagnosis of osteoporosis and should trigger further assessment and treatment if necessary.
Conventional X-rays are the first step of the diagnostic work-up to detect osteoporotic fracture. Simple X-rays can easily detect the presence of fractures, particularly in the upper and lower extremities, but occult fractures at any site are very difficult to diagnose on simple X-ray. If occult fracture is highly suspected on history and physical examination, computed tomography (CT), bone scintigraphy (BS), or magnetic resonance imaging (MRI) are recommended as additional diagnostic tools to confirm osteoporotic fracture.
In general, osteoporotic fractures of the vertebrae and the pelvic ring and their severity are often underdiagnosed or underestimated on conventional X-rays because of inadequate film quality, lack of fracture recognition by radiologists, or use of ambiguous terminology in reports.[40414243]
In most fractures of the pelvic ring, conventional X-rays mainly detect ventral pelvic fractures; therefore, additional CT is necessary to evaluate the dorsal pelvis. Especially in cases in which osteoporotic fracture of the pelvic ring is strongly suspected without an obvious fracture line on conventional X-rays, MRI of the pelvis is of great help and may be a gold standard.[4041]
Osteoporotic vertebral fractures are frequently undetected, but their accurate and early diagnosis is of paramount importance because both symptomatic and asymptomatic vertebral fractures are associated with increased morbidity and mortality.[42] Preferentially, conventional X-rays are crucial for diagnosing and grading osteoporotic vertebral deformities and for differential diagnosis during assessment for osteoporotic deformity. However, more advanced imaging techniques such as CT, BS, and MRI may be required to further investigate the etiology in some of these deformities as well as to differentiate chronic from acute fractures, to assess compromise of the spinal canal, and for follow-up after specific treatments such as vertebroplasty.[43]
Osteoporotic fractures are a frequent and important cause of disability and medical costs worldwide. Moreover, a number of osteoporotic fractures such as hip and vertebral fractures have very high morbidity and mortality.[28] Prior osteoporotic fracture at any site is one of the strongest risk factors for a new fracture, partly independent of BMD [44]; The new fractures occur very soon after the first fracture. Therefore, early and accurate diagnosis of osteoporotic fractures using the clinical definition, which is applicable practically and independent of BMD, is crucial for preventing subsequent fractures and reducing their associated socioeconomic burden. Fractures caused by low-level trauma equivalent to a fall from a standing height or less at sites described above in adults over age 50, should be first regarded as osteoporotic. In addition, if osteoporotic fractures are strongly suspected on history and physical examination even though there are no positive findings on conventional X-rays, more advanced imaging techniques such as CT, BS, and MRI are required. Subsequently, appropriate treatment should be administered as soon as possible after the diagnosis of the first osteoporotic fracture in order to be most cost-effective.
Figures and Tables
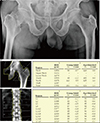 | Fig. 1A fracture of the right femoral neck in a 76-year-old male patient is shown on a preoperative radiograph. The fracture was caused by a simple fall from a bed. There was no finding of osteoporosis on dual energy X-ray absorptiometry measured at the proximal femur and lumbar spine. |
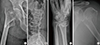 | Fig. 2Typical osteoporotic fractures at major sites. (A) Hip, (B) Spine, (C) Distal radius, (D) Proximal humerus. |
References
1. Yi H, Ha YC, Lee YK, et al. National healthcare budget impact analysis of the treatment for osteoporosis and fractures in Korea. J Bone Metab. 2013; 20:17–23.

2. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004; 34:195–202.

3. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006; 17:1726–1733.

4. Lee KM, Chung CY, Kwon SS, et al. Ankle fractures have features of an osteoporotic fracture. Osteoporos Int. 2013; 24:2819–2825.

5. Soles GL, Ferguson TA. Fragility fractures of the pelvis. Curr Rev Musculoskelet Med. 2012; 5:222–228.

6. Sajjan SG, Barrett-Connor E, McHorney CA, et al. Rib fracture as a predictor of future fractures in young and older postmenopausal women: National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2012; 23:821–828.

7. Ng AC, Drake MT, Clarke BL, et al. Trends in subtrochanteric, diaphyseal, and distal femur fractures, 1984-2007. Osteoporos Int. 2012; 23:1721–1726.

8. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996; 312:1254–1259.

9. Ha YC, Park YG, Nam KW, et al. Trend in hip fracture incidence and mortality in Korea: a prospective cohort study from 2002 to 2011. J Korean Med Sci. 2015; 30:483–488.

10. Rowe SM, Song EK, Kim JS, et al. Rising incidence of hip fracture in Gwangju City and Chonnam Province, Korea. J Korean Med Sci. 2005; 20:655–658.

11. Rowe SM, Yoon TR, Ryang DH. An epidemiological study of hip fracture in Honam, Korea. Int Orthop. 1993; 17:139–143.

12. Park C, Ha YC, Jang S, et al. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab. 2011; 29:744–751.

13. Meema HE, Meema S. Cortical bone mineral density versus cortical thickness in the diagnosis of osteoporosis: a roentgenologic-densitometric study. J Am Geriatr Soc. 1969; 17:120–141.

14. Keshawarz NM, Recker RR. Expansion of the medullary cavity at the expense of cortex in postmenopausal osteoporosis. Metab Bone Dis Relat Res. 1984; 5:223–228.

15. Ahlborg HG, Johnell O, Turner CH, et al. Bone loss and bone size after menopause. N Engl J Med. 2003; 349:327–334.

16. Haara M, Heliövaara M, Impivaara O, et al. Low metacarpal index predicts hip fracture: a prospective population study of 3,561 subjects with 15 years of follow-up. Acta Orthop. 2006; 77:9–14.

17. Mather J, MacDermid JC, Faber KJ, et al. Proximal humerus cortical bone thickness correlates with bone mineral density and can clinically rule out osteoporosis. J Shoulder Elbow Surg. 2013; 22:732–738.

18. Roberts M, Yuan J, Graham J, et al. Changes in mandibular cortical width measurements with age in men and women. Osteoporos Int. 2011; 22:1915–1925.

19. Sadat-Ali M, Elshaboury E, Al-Omran AS, et al. Tibial cortical thickness: A dependable tool for assessing osteoporosis in the absence of dual energy X-ray absorptiopmetry. Int J Appl Basic Med Res. 2015; 5:21–24.

20. Johannesdottir F, Turmezei T, Poole KE. Cortical bone assessed with clinical computed tomography at the proximal femur. J Bone Miner Res. 2014; 29:771–783.

21. Bala Y, Zebaze R, Ghasem-Zadeh A, et al. Cortical porosity identifies women with osteopenia at increased risk for forearm fractures. J Bone Miner Res. 2014; 29:1356–1362.

22. Lenchik L, Rogers LF, Delmas PD, et al. Diagnosis of osteoporotic vertebral fractures: importance of recognition and description by radiologists. AJR Am J Roentgenol. 2004; 183:949–958.

23. WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994; 843:1–129.
24. Kanis JA, Melton LJ 3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994; 9:1137–1141.

25. World Health Organization. Assessment of osteoporosis at the primary health care level. Summary Report of a WHO Scientific Group. 2007. www.who.int/chp/topics/rheumatic/en/index.
26. Hey HW, Sng WJ, Lim JL, et al. Interpretation of hip fracture patterns using areal bone mineral density in the proximal femur. Arch Orthop Trauma Surg. 2015; 135:1647–1653.

27. Kanis JA, Oden A, Johnell O, et al. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001; 12:417–427.

28. Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005; 16:Suppl 2. S3–S7.

29. Nguyen TV, Eisman JA, Kelly PJ, et al. Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol. 1996; 144:255–263.

30. McLellan AR, Gallacher SJ, Fraser M, et al. The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int. 2003; 14:1028–1034.

31. Tsiridis E, Upadhyay N, Giannoudis PV. Sacral insufficiency fractures: current concepts of management. Osteoporos Int. 2006; 17:1716–1725.

32. Palvanen M, Kannus P, Niemi S, et al. Hospital-treated minimal-trauma rib fractures in elderly Finns: long-term trends and projections for the future. Osteoporos Int. 2004; 15:649–653.

33. Barrett-Connor E, Nielson CM, Orwoll E, et al. Epidemiology of rib fractures in older men: Osteoporotic Fractures in Men (MrOS) prospective cohort study. BMJ. 2010; 340:c1069.

34. Biver E, Durosier C, Chevalley T, et al. Prior ankle fractures in postmenopausal women are associated with low areal bone mineral density and bone microstructure alterations. Osteoporos Int. 2015; 26:2147–2155.

35. Koski AM, Patala A, Patala E, et al. Incidence of osteoporotic fractures in elderly women and men in Finland during 2005-2006: a population-based study. Scand J Surg. 2014; 103:215–221.

36. Johnell O. The socioeconomic burden of fractures: today and in the 21st century. Am J Med. 1997; 103:20S–25S.

37. Melton LJ 3rd, Chrischilles EA, Cooper C, et al. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992; 7:1005–1010.

38. National Osteoporosis Foundation. Osteoporosis Disease Statistics. 2006. cited by 2008 October 3. Available from: http://www.nof.org/osteoporosis/stats.htm.
39. Tromp AM, Ooms ME, Popp-Snijders C, et al. Predictors of fractures in elderly women. Osteoporos Int. 2000; 11:134–140.

40. Rommens PM, Wagner D, Hofmann A. Surgical management of osteoporotic pelvic fractures: a new challenge. Eur J Trauma Emerg Surg. 2012; 38:499–509.

41. Rommens PM, Ossendorf C, Pairon P, et al. Clinical pathways for fragility fractures of the pelvic ring: personal experience and review of the literature. J Orthop Sci. 2015; 20:1–11.

42. Delmas PD, van de Langerijt L, Watts NB, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res. 2005; 20:557–563.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download