Abstract
Background
This study was conducted to observe the prevalence of vitamin D deficiency during pregnancy and the effects of maternal 25-hydroxy-vitamin D (25-[OH]D) levels on fetal bone growth.
Methods
Five hundred twenty-three Korean pregnant women were randomly recruited and serum 25-(OH)D level was measured. During pregnancy, fetal ultrasonography and serum 25-(OH)D measurements were carried out 3 times in 275 of 523 pregnant women. Fetal biparietal and occipitofrontal diameter, head and abdominal circumference, and femur and humerus length were measured through fetal ultrasonography.
Results
The prevalence of vitamin D deficiency (25-[OH]D<20 ng/mL) based on the 1st serum measurement of 25-(OH)D was 88.9%. There was no association between maternal serum 25-(OH)D level and fetal bone growth. In 275 pregnant women who completed study design, the mean value of 25-(OH)D was 12.97±5.93, 19.12±9.82, and 19.60±9.98 ng/mL at 12 to 14, 20 to 22, and 32 to 34 weeks of pregnancy, respectively and there was an association between the difference of serum 25-(OH)D level between 12 to 14 and 20 to 22 weeks and growth velocity of fetal biparietal diameter between 20 to 22 and 32 to 34 weeks of pregnancy.
Vitamin D has an important role in bone metabolism and also in non-skeletal systems such as muscle, immunefunction, cardiovascular system, insulin metabolism, and skin.[12] Maternal vitamin D inadequacy may have adverse effects on the mother and the offspring.[345] Suboptimal maternal vitamin D status is associated with several adverse pregnancy outcomes, including pre-eclampsia,[6] fetal growth retardation, preterm labor,[7] increased rate of primary cesarean section [8] and gestational diabetes mellitus (GDM).[9] Although optimal vitamin D status is important during pregnancy, the cutoff for optimal serum 25-hydroxy-vitamin D (25-[OH]D) levels during pregnancy remains controversial. At present, it is assumed that the optimal levels in pregnancy are the same as in the general population.[1011] Despite the reported high prevalence of deficiency and the possible consequences, the desired optimal level for pregnant women, the amount of vitamin D intake required to maintain adequate levels and the role of vitamin D during pregnancy are poorly documented.[1213]
Although vitamin D deficiency among pregnant women is common worldwide, data regarding the prevalence of vitamin D deficiency and the effects that it has on fetuses in Korea are sparse. We prospectively observed the prevalence of vitamin D deficiency, and the association between the changes in serum 25-(OH)D during pregnancy and the effects of serum 25-(OH)D levels on fetal bone growth.
Early-term pregnant women who visited the Department of Obstetrics at Cheil General Hospital & Women's Healthcare Center, Dankook University, were randomly recruited from May, 2011 to July, 2012. Five hundred twenty-three Korean pregnant women were registered after obtaining written informed consent. Inclusion criteria were early pregnant Korean women who agree to participate this study. Exclusion criteria for participation were 1) women who have twin babies as pregnancy progressed and 2) women who have any diseases that could affect bone metabolism.
Blood samples were collected at 12 to 14 weeks, 20 to 22 weeks, and 32 to 34 weeks for 25-(OH)D measurement. Serum 25-(OH)D levels were measured using an electrochemiluminescence immunoassay (ECLIA) method on the Roche Modular Analytics E170 (Roche Diagnostics, Mannheim, Germany). The intra-assay coefficients of variation were 2.4% at 40.48 ng/mL.
During pregnancy, fetal ultrasonography was carried out 3 times (at 12 to 14 weeks, 20 to 22 weeks, and 32 to 34 weeks). Fetal biparietal and occipitofrontal diameter, head and abdominal circumference, and femur and humerus length were measured through fetal ultrasonography. To calculate fetal growth, the differences in length between the 1st and 2nd and the 2nd and 3rd ultrasonographys were divided by the number of days between ultrasonography examinations. Assessments were performed by obstetric radiologists using a Voluson E8 ultrasound (GE Medical Systems, Milwaukee, WI, USA). The ultrasonography and inter-sonography intervals were all based on routine clinical practice and not standardized as part of this study protocol. The study protocol was approved by the Institutional Review of Board of Cheil General Hospital on January 31, 2011 (CGH-IRB-2-11-2).
Analyses were performed by using SAS software, version 9.3 (SAS Institute, Cary, NC, USA). A mixed model was applied to repeated measurements of 25-(OH)D after natural logarithm transformation due to non-normality. For repeated measurement of binary outcome of 25-(OH)D, the data was analyzed using generalized estimating equation (GEE). P-values were corrected by Bonferroni's method in the case of multiple testing, and were considered significant at 0.05. Data are expressed as means±standard deviation (SD). Comparisons between groups in 275 pregnant women who completed study design were performed by a two-sampled t-test. Pearson correlation coefficient was used to observe the correlation between variables.
The study design is shown in Figure 1. Characteristics of enrolled pregnant women such as age, body mass index (BMI), seasons of blood collection, and number of delivery were shown in Table 1. Serum 25-(OH)D levels at the time of first collection did not associate with age, BMI and number of delivery (data not shown). In total, 515, 388 and 304 pregnant women were carried out serum 25-(OH)D measurements at 12 to 14 weeks, 20 to 22 weeks, and 32 to 34 weeks, respectively. Four hundred thirty-nine pregnant women received a fetal ultrasonography.
The prevalence of 25-(OH)D deficiency (<20 ng/mL) during pregnancy was 88.9% at 12 to 14 weeks of pregnancy in 523 pregnant women. The prevalence of vitamin D deficiency decreased as pregnancy progressed. Two-hundred forty-eight subjects did not complete all three blood samples and ultrasonographys. The mean values of the 2nd and 3rd 25-(OH)D measurements were significantly different from the 1st 25-(OH)D value, even after adjusting for season. Although 25-(OH)D levels at 20 to 22 and 32 to 34 weeks were increased compared with 12 to 14 weeks, the overall mean level of 25-(OH)D was still below 20 ng/mL (Table 2).
There was no association between maternal serum 25-(OH)D level with growth velocity of fetal biparietal and occipitofrontal diameter, head and abdominal circumference, femur and humerus length (Table 3). There was no difference in fetal growth between pregnant women whose serum 25-(OH)D level was higher than 20 ng/mL and those who were 25-(OH)D deficient (Fig. 2).
Two hundred forty-five pregnant women were completed the blood samplings and untrasonograms at 12 to 14, 20 to 22, and 32 to 34 weeks of pregnancy. The mean value of 25-(OH)D at 12 to 14, 20 to 22, and 32 to 34 weeks of pregnancy was 12.97±5.93, 19.12±9.82, and 19.60±9.98 ng/mL, respectively (Fig. 3). Changes of 25-(OH)D was similar to those of 523 enrolled pregnant women in this study. Analysis of data in 275 pregnant women showed that there was a negative correlation between serum 25-(OH)D level at 12 to 14 weeks and growth velocity of fetal biparietal diameter between 20 to 22 and 32 to 34 weeks of pregnancy, however, there was a positive correlation between the difference of serum 25-(OH)D levels between 12 to 14 and 20 to 22 weeks and growth velocity of fetal biparietal diameter between 20 to 22 and 32 to 34 weeks of pregnancy (Fig. 4). Other parameters such as fetal occipitofrontal diameter, head and abdominal circumference, femur and humerus length were not associated with serum 25-(OH)D and the difference of 25-(OH)D levels during pregnancy.
Studies on vitamin D and fetal growth are still limited, and a recent review of maternal vitamin D and fetal growth in Europe and the United States did not produce consistent results.[14] This study prospectively observed the prevalence of vitamin D deficiency and the association between serum 25-(OH)D and fetal growth in Korean pregnant women by measuring serum 25-(OH)D and carrying out fetal ultrasonography three times during pregnancy. Vitamin D deficiency (25-[OH]D concentration <20 ng/mL) is common inpregnant women throughout the world.[12] This study showed that vitamin D deficiency in Korean pregnant women was also common, and the prevalence was 88.9% at 12 to 14 weeks of pregnancy. The change in 25-(OH)D level exhibited during pregnancy in this study was different from the previous results, which reported increases,[15] declines [16] or no change.[17] In this study, we observed that serum 25-(OH)D levels increased as pregnancy progressed, after adjustment for seasonal variations, but the average serum level of 25-(OH)D was still lower than 20 ng/mL. A plausible explanation is the nonclassical roles of vitamin D in pregnancy since the placenta is probably the most prominent site for extra-renal production of vitamin D.[18] Other probable explanation is that increasing 25-(OH)D levels may be due to an increase in vitamin D-binding protein (DBP), since DBP binds 80% to 95% of serum 25-(OH)D in pregnancy.[19] Further investigation is required to define an appropriate vitamin D deficiency cutoff and to explain the mechanism of 25-(OH)D increase during pregnancy.
Vitamin D deficiency is known to affect fetal bone growth during pregnancy.[20] Preeclampsia, gestational diabetes, impaired fetal growth, immune intolerance, and low birth weight may be related to vitamin D deficiency during pregnancy.[10] However, definitive evidence of the association between vitamin D deficiency and problems related to pregnancy is still lacking. We measured maternal serum 25-(OH)D and fetal biparietal and occipitofrontal diameter, head and abdominal circumference, and femur and humerus length via ultrasonography three times during pregnancy and calculated fetal growth velocity in order to observe the association between serum 25-(OH)D and differences of 25-(OH)D levels at each stages of pregnancy with fetal growth. Since maternal vitamin D status may influence fetal bone health [2021] and the prevalence of vitamin D deficiency in Korean women is higher than in other races,[22] we hypothesized that the higher prevalence of vitamin D deficiency was related to the risk of health problems for the mother and fetus during pregnancy. However, maternal serum 25-(OH)D levels did not affect fetal bone growth during pregnancy despite of the high prevalence of vitamin D deficiency in pregnant Korean women. In contrast, a negative correlation between serum 25-(OH)D level at 12 to 14 weeks and growth velocity of fetal biparietal diameter between 20 to 22 and 32 to 34 weeks of pregnancy, and a positive correlation between the difference of serum 25-(OH)D levels between 12 to 14 and 20 to 22 weeks and growth velocity of fetal biparietal diameter between 20 to 22 and 32 to 34 weeks were observed, we speculate that although initial serum 25-(OH)D level is low, the changes of 25-(OH)D levels between 12 to 14 and 20 to 22 weeks are important for growth of fetal biparietal diameter. However, we could not explain why other parameters such as fetal occipitofrontal diameter, head and abdominal circumference, and femur and humerus length were not associated with the changes of 25-(OH)D levels. Additional studies are warranted to observe the effects of 25-(OH)D on fetal growth.
We verified the relationship between vitamin D and fetal bone growth by using fetal ultrasonography and measured 25-(OH)D three times from the early stage of pregnancy until childbirth. One of the limitations of this study is that, because the pregnant women in this study were recruited from a single hospital, the results may not be generalizable to all pregnant women in Korea. However, since the pregnant women who participated in this study were randomly selected from a hospital with approximately 500 deliveries per month, this limitation might be overcome. Secondly, we did not measure DBP and bioavailable 25-(OH)D. While different levels of total 25-(OH)D and DBP were reported between African Americans and whites, bioavailable 25-(OH)D was similar,[23] thus, we could not figure out whether bioavailable 25-(OH)D level was deficient or not in this study subjects. Thirdly, all enrolled pregnant women did not complete serum 25-(OH)D measurement and ultrasonography three times because they missed blood sampling and ultrasonography on a scheduled time without any health problems. Therefore, a mixed model was applied to repeated measurements of 25-(OH)D for statistical analysis because of missing data. There were 13 cases of abortion among the subjects, but no correlation with vitamin D deficiency was observed (data not shown). And three women were excluded during pregnancy because of twin babies
In conclusion, although we did not observe any associations of maternal 25-(OH)D with fetal growth except one association between the changes of serum 25-(OH)D levels and the growth of fetal biparietal diameter despite of a high prevalence of vitamin D deficiency in this study, the results of this study should not be interpreted as suggesting that 25-(OH)D levels are not an important determinants during pregnancy until future studies show new data. Further studies are necessary to define vitamin D deficiency and elucidate the relationship between vitamin D level and pregnancy.
Figures and Tables
Fig. 1
Flow of measurements for participants in this study. 275 among 523 early pregnant women were completed the blood samplings and ultrasonographys three times. 25-(OH)D, 25-hydroxy-vitamin D. Parameters for measurement. *biparietal diameter (BPD), abdominal circumference (AC); **biparietal diameter (BPD), occipitofrontal diameter (OFD), head circumference (HC), abdominal circumference (AC) femur length (FL), humerus length (HL).
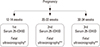
Fig. 2
Comparison of fetal growth velocity between pregnant women whose serum 25-(OH)D level was higher than 20 ng/mL and those who were 25-(OH)D deficient. (A) No association between serum 25-(OH)D levels at 12 to 14 weeks and the growth velocity between 12 to 14 and 20 to 22 weeks of pregnancy was observed. (B) The growth velocity between 12 to 14 and 20 to 22 weeks and between 20 to 22 and 32 to 34 weeks of pregnancy were not associated with serum 25-(OH)D levels at 20 to 22 weeks. (C) No association between serum 25-(OH)D levels at 32 to 34 weeks and the growth velocity between 20 to 22 and 32 to 34 weeks of pregnancy was observed. 25-(OH)D, 25-hydroxy-vitamin D; BPD, biparietal diameter; AC, abdominal circumference; OFD, occipitofrontal diameter; HC, head circumference; FL, femur length; HL, humerus length. BPD (1), AC(1) : growth velocity between 12-14 and 20-22 weeks of pregnancy. BPD(2), AC(2), OFD(2), HC(2), FL(2), HL(2): growth velocity between 20-22 and 32-34 weeks of pregnancy.

Fig. 3
Mean value of serum 25-(OH)D at 12 to 14, 20 to 22, 32 to 34 weeks of pregnancy in 275 pregnant women who completed study design. Mean value of serum 25-(OH)D at 12 to 14 weeks was significantly different from the mean value at 20 to 22 and 32 to 34 weeks of pregnancy. 25-(OH)D, 25-hydroxy-vitamin D.

Fig. 4
Correlation of the difference of serum 25-(OH)D levels between 12 to 14 and 20 to 22 weeks with the growth velocity of fetal biparietal diameter between 20 to 22 and 32 to 34 weeks of pregnancy in 275 pregnant women who completed study design. A positive correlation was observed between the difference of serum 25-(OH)D levels between 12 to 14 and 20 to 22 weeks and growth velocity of fetal biparietal diameter between 20 to 22 and 32 to 34 weeks of pregnancy. 25-(OH)D, 25-hydroxy-vitamin D.

Notes
References
1. Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008; 29:361–368.

3. Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr. 2011; 31:89–115.

4. Lewis S, Lucas RM, Halliday J, et al. Vitamin D deficiency and pregnancy: from preconception to birth. Mol Nutr Food Res. 2010; 54:1092–1102.

5. Lapillonne A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med Hypotheses. 2010; 74:71–75.

6. Bodnar LM, Catov JM, Simhan HN, et al. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007; 92:3517–3522.

7. Morley R, Carlin JB, Pasco JA, et al. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006; 91:906–912.

8. Merewood A, Mehta SD, Chen TC, et al. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009; 94:940–945.

9. Dawodu A, Akinbi H. Vitamin D nutrition in pregnancy: current opinion. Int J Womens Health. 2013; 5:333–343.

10. Mulligan ML, Felton SK, Riek AE, et al. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010; 202:429.e1–429.e9.

11. Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutr Rev. 2010; 68:465–477.

12. Wagner CL, Taylor SN, Dawodu A, et al. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients. 2012; 4:208–230.

13. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–1930.

14. Galthen-Sørensen M, Andersen LB, Sperling L, et al. Maternal 25-hydroxyvitamin D level and fetal bone growth assessed by ultrasound: a systematic review. Ultrasound Obstet Gynecol. 2014; 44:633–640.

15. Cross NA, Hillman LS, Allen SH, et al. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995; 61:514–523.

16. Zhang JY, Lucey AJ, Horgan R, et al. Impact of pregnancy on vitamin D status: a longitudinal study. Br J Nutr. 2014; 112:1081–1087.

17. Bouillon R, Van Assche FA, Van Baelen H, et al. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981; 67:589–596.

18. Novakovic B, Sibson M, Ng HK, et al. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J Biol Chem. 2009; 284:14838–14848.
19. Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013; 369:1991–2000.

20. Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010; 95:1749–1757.

21. Mahon P, Harvey N, Crozier S, et al. Low maternal vitamin D status and fetal bone development: cohort study. J Bone Miner Res. 2010; 25:14–19.

22. Hamilton SA, McNeil R, Hollis BW, et al. Profound Vitamin D Deficiency in a Diverse Group of Women during Pregnancy Living in a Sun-Rich Environment at Latitude 32 degrees N. Int J Endocrinol. 2010; 2010:917428.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download