Abstract
Background
The purpose of this study was to find out the cause of discrepancy between various automated immunoassays for 25-hydroxy-vitamin D (25-[OH]D).
Methods
National Institute of Standards & Technology Standard Reference Material (SRM) 972a is SRM for 25-(OH)D and consists of 4 vials of frozen serum with different concentrations of 25-(OH)D. Each concentration was measured 6 times in 3 different immunoassays: ADVIA Vitamin D Total assay (Siemens Healthcare, Erlangen, Germany), ARCHITECT 25-(OH)D (Abbott Laboratories, Abbott Park, IL, USA), and COBAS Vitamin D Total assay (Roche Diagnostics, Basel, Switzerland).
Results
When using the certified reference values of SRM 972a as it is, discarding the cross-reactivity of each immunoassay, for ADVIA, the coefficient of determination (R2) as a score of regression analysis was 0.8995 and maximal difference between measured value and certified reference value was 3.6 ng/mL in level 3. The R2 and maximal differences of ARCHITECT were 0.5377 and 6.9 ng/mL, respectively, in level 4. Those of COBAS were 0.3674 and 22.3 ng/mL, respectively, in level 4. When considering cross-reactivities of each immunoassays to various 25-(OH)D metabolites, the ADVIA had R2 and maximal difference of 0.9254 and 3.3 ng/mL, respectively, in level 3. For ARCHITECT, the R2 and maximal differences were 0.7602 and 5.1 ng/mL, respectively, in level 1. Those of COBAS were 0.9284 and 4.9 ng/mL, respectively, in level 1.
Vitamin D, a fat-soluble vitamin, is well known for its important role in maintaining calcium homeostasis, promoting bone formation in children, and maintaining bone strength in adults.[1] Vitamin D deficiency in children is the main cause of skeletal deformities such as rickets; and vitamin D deficiency in elderly could be the cause of osteoporosis and sarcopenia.[12]
Some studies have shown that higher serum levels of vitamin D can reduce the incidence of cancers such as breast, colorectal, and prostate.[3] Recent studies reported that low 25-hydroxy-vitamin D (25-[OH]D) levels are associated with the cardiovascular disease risk factors of hypertension, obesity, diabetes mellitus and
the metabolic syndrome, as well as cardiovascular disease events including stroke and congestive heart failure.[4]
Vitamin D exists in 2 major forms, Vitamin D2 (ergocalciferol) and D3 (cholecalciferol). These are then metabolized to 25-(OH)D2 and 25-(OH)D3 in the liver. Measuring the levels of both 25-(OH)D2 and 25-(OH)D3 is imperative to the assessment of the clinical nutritional status.[5] Vitamin D2 or D3 is provided as a vitamin D supplement in many countries.
Measurement of 25-(OH)D is performed with a number of different analytical techniques. Serum 25-(OH)D levels can be measured by radioimmunoassay (RIA), enzyme immunoassay (EIA), chemiluminescent immunoassay (CLIA), electro-CLIA (ECLIA), high-performance liquid chromatography (HPLC), and by the recently developed liquid chro-matography-tandem mass spectrometry (LC-MS/MS) technique.[6] Recently, automated 25-(OH)D immunoassays facilitate rapid measurement of serum vitamin D levels.[78] Of the several diagnostic companies, Siemens, Roche, and Abbott provide automated 25-(OH)D assays. However, the results of automated 25-(OH)D assays are inconsistent and difficult to compare. Therefore, the assays for vitamin D need to be standardized. The purpose of this study was to find out the cause of discrepancy between various automated immunoassays for 25-(OH)D using certified reference material.
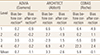
Standard Reference Material (SRM) 972a (National Institute of Standards and Technology [NIST], Gaithersburg, MD, USA) is the standard reference material for vitamin D. It is intended for use as an accuracy control in the critical evaluation of methods for determining the amount-of-substance concentration of vitamin D metabolites in human serum.[9] One unit of SRM 972a consists of 4 vials (Level 1 through 4) of frozen serum with different concentration levels of 25-(OH)D (Table 1). Levels 1, 2, and 3 of SRM 972a were prepared from pools of human serum with endogenous concentrations of vitamin D metabolites. Level 4 was prepared from a pool of human serum fortified with 3-epi-25-(OH)D3.
The 3 automated immunoassays for 25-(OH)D used in this study were ADVIA Centaur Vitamin D Total (Siemens Healthcare, Erlangen, Germany), ARCHITECT 25-OH Vitamin D (Abbott Laboratories, Abbott Park, IL, USA), and COBAS Vitamin D Total (Roche Diagnostics, Basel, Switzerland). These assays use CLIA or ECLIA techniques. They measure various vitamin D metabolites, mainly 25-(OH)D2, 25-(OH)D3, and selectively 3-epi-25-(OH)D3, and then report the sum as total 25-(OH)D.
For the measurement in 3 different immunoassays, we used 2 units of SRM 972a. Using first unit of SRM 972a, we performed 3 repeated measurements in each immunoassay and repeated the same procedure using second unit of SRM 972a. We thus performed a total of 6 measurements for the combination of each immunoassay and each level of SRM 972a.
First, we assessed the biases of each immunoassay with measured values and certified values. Second, we assessed the biases of each immunoassay with corrected certified values for cross-reactivity.
Correction for cross-reactivity of each immunoassay was performed as follows:
Corrected certified value of 25-(OH)D in an immunoassay=Σ(Certified value of SRM 972a×Cross reactivity of the immunoassay about specific metabolites)
Specific metabolites: 25-(OH)D2, 25-(OH)D3, 3-epi-25-(OH)D3
Cross-reactivities of specific metabolites in each immunoassay were provided by each manufacturer and presented in Table 2.
In the comparison between measured values and certified reference values of SRM 972a, the averages of measured values in 3 automated immunoassays were presented in Table 3. The average bias was presented in Table 4 as 'Bias before correction'. That of ADVIA (Siemens) was 0.7 ng/mL (Min -0.7, Max 3.6). That of ARCHITECT (Abbott) was 3.3 ng/mL (Min -2.9, Max 6.9) and that of COBAS (Roche) was 5.8 ng/mL (Min -5.6, Max 22.3). Regression plot, equation for regression line, and coefficient of determination (R2) between measured values and certified reference values were shown in Figure 1A, C, and E.
When we corrected the certified reference values of SRM 972a considering cross-reactivity of each immunoassay, the average bias changed to the values of 'Bias after correction', as shown in Table 4. That of ADVIA (Siemens) was -1.1 ng/mL (Min -3.3, Max 0.2). That of ARCHITECT (Abbott) was -2.6 ng/mL (Min -5.1, Max 1.5) and that of COBAS (Roche) significantly decreased to -0.1 ng/mL (Min -4.9, Max 2.4). The R2 in each regression analysis was improved from 0.8995 to 0.9254 in ADVIA (Siemens), from 0.5377 to 0.7602 in ARCHITECT (Abbott), and from 0.3674 to 0.9284 in COBAS (Roche) (Fig. 1).
The clinical importance of vitamin D is gaining emphasis.[123410111213] Discrepancies between vitamin D total immunoassays are constantly raised and reported in the several studies.[781415] Various causes of the discrepancies have been referred,[16] but so far, the clear cause of discrepancies between immunoassays was unknown. In this study, we focused on the cross-reactivities with various vitamin D metabolites and confirmed that difference in cross-reactivities may be the main cause of discrepancy between immunoassays. Especially, the bias before considering cross-reactivity was greatest in COBAS (Roche) and the reason was the relatively high cross-reactivity (91% in Table 2) to the epimer form of vitamin D3, 3-epi-25-(OH)D3. Though the epimer, 3-epi-25-(OH)D3, is known to have significantly reduced calcemic effect, as compared with 25-(OH)D3,[1718] it accounts for a significant proportion of total 25-(OH)D in adults [19] as well as infants.[20] Therefore, it is important to discriminated the epimer from total 25-(OH)D.
Due to the discrepancies of various vitamin D assays, the need for standardization has been further highlighted. Generally, there are 2 possible ways for standardization. The first is using a reference measurement procedure. A method using isotope-dilution (ID) LC-MS/MS was developed and has been accepted as a reference method.[521] However, typical clinical laboratories are not equipped for mass spectrometry because of high cost and difficulty of operation. Second, the possible alternative is to use relatively cheap standard reference material. However, as the results of this study indicated, the use of certified reference values as it is, results in large differences with measured values in a certain immunoassay. For successful measurement of standard reference material, we must consider the cross-reactivity of each immunoassay. But, even considering cross-reactivities of each immunoassay, it is not sufficient for the standardization of total vitamin D immunoassays. These assays have intrinsic and indelible limitation for standard-ization. Because the analyte of total vitamin D assay is not a pure substance but mixture of various metabolites and the mixing proportion of these metabolites is diverse in each individual, it is difficult to specify a certain correction factor for the standardization.
The limitation of this study was that the sample was not a natural human serum. Though the reference material was based on human serum, the detailed composition of vitamin D metabolites can be different from natural human serum. Especially, 3-epi-25-(OH)D3 was fortified in the SRM 972a, hence the proportion of epimer may be different to the natural human serum. In additional research, it is necessary to verify the actual effect of cross-reactivities using various natural human serum.
In summary, significant discrepancies between measured values and certified reference values were found in the 25-(OH)D immunoassays. The cause of discrepancies was on the difference in cross-reactivities to various vitamin D metabolites. And the discrepancies can be considerably decreased by considering cross-reactivities of each immunoassay.
Figures and Tables
 | Fig. 1Regression plots between reference values and measured values of Standard Reference Material (SRM) 972a. (A) and (B) are of ADVIA (Siemens), (C) and (D) are of ARCHITEC (Abbott), (E) and (F) are of COBAS (Roche). X axis means reference values of SRM 972a in (A), (C), and (E); while X axis means corrected reference values for cross-reactivity in (B), (D), and (F). |
References
1. Bentley J. The role of vitamin D in infants, children and young people. Nurs Child Young People. 2015; 27:28–35.

2. Meehan M, Penckofer S. The Role of Vitamin D in the Aging Adult. J Aging Gerontol. 2014; 2:60–71.

3. Garland CF, Gorham ED, Mohr SB, et al. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009; 19:468–483.

4. Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care. 2008; 11:7–12.

5. Saenger AK, Laha TJ, Bremner DE, et al. Quantification of serum 25-hydroxy-vitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006; 125:914–920.

6. Kwak HS, Chung HJ, Cho DH, et al. Efficacy of the measurement of 25-hydroxy-vitamin D2 and D3 levels by using PerkinElmer liquid chromatography-tandem mass spectrometry vitamin D kit compared with DiaSorin radioimmunoassay kit and Elecsys vitamin D total assay. Ann Lab Med. 2015; 35:263–265.

7. Farrell CJ, Martin S, McWhinney B, et al. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012; 58:531–542.

8. Janssen MJ, Wielders JP, Bekker CC, et al. Multicenter comparison study of current methods to measure 25-hydroxy-vitamin D in serum. Steroids. 2012; 77:1366–1372.

9. Gonzalez CA, Watters RL Jr. Certificate of analysis. Standard Reference Material® 972a: Vitamin D metabolites in frozen human serum. Gaithersburg, MD: National Institute of Standards & Technology;2013.
11. Zhang R, Naughton DP. Vitamin D in health and disease: current perspectives. Nutr J. 2010; 9:65.

12. Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011; 31:48–54.

13. Kim MK, Baek KH, Song KH, et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the Fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab. 2011; 96:3250–3256.

14. Carter GD. Accuracy of 25-hydroxy-vitamin D assays: confronting the issues. Curr Drug Targets. 2011; 12:19–28.

15. Farrell C, Soldo J, Williams P, et al. 25-Hydroxyvitamin D testing: challenging the performance of current automated immunoassays. Clin Chem Lab Med. 2012; 50:1953–1963.

16. Fuleihan Gel H, Bouillon R, Clarke B, et al. Serum 25-Hydroxyvitamin D Levels: Variability, Knowledge Gaps, and the Concept of a Desirable Range. J Bone Miner Res. 2015; 30:1119–1133.

17. Brown AJ, Ritter C, Slatopolsky E, et al. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha, 25-dihydroxy-vitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem. 1999; 73:106–113.

18. Fleet JC, Bradley J, Reddy GS, et al. 1 alpha,25-(OH)2-vitamin D3 analogs with minimal in vivo calcemic activity can stimulate significant transepithelial calcium transport and mRNA expression in vitro. Arch Biochem Biophys. 1996; 329:228–234.

19. Strathmann FG, Sadilkova K, Laha TJ, et al. 3-epi-25 hydroxy-vitamin D concentrations are not correlated with age in a cohort of infants and adults. Clin Chim Acta. 2012; 413:203–206.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download