Abstract
Osteopetrosis is a rare genetic bone disease characterized by increased bone density but prone to breakage due to defective osteoclastic function. Among two primary types of autosomal dominant osteopetrosis (ADO), osteopetrosis type II is characterized by sclerosis of bones, predominantly involving the spine, the pelvis, and the skull base. Fragility of bones and dental abscess are leading complications. This report presents a case of osteopetrosis in a 52-years-old female, which was complicated by the development of cavernous sinus thrombophlebitis and meningitis. She was suffered from multiple fractures since one year ago. Laboratory data revealed elevated serum levels of tartrate resistant acid phosphatase (TRAP) without carbonic anhydrase II DNA mutation. A thoracolumbar spine X-ray showed, typical findings of ADO type II (ADO II; Albers-Schönberg disease), prominent vertebral endplates so called the 'rugger jersey spine'. Her older sister also showed same typical spine appearance. We report a case of ADO II with cavernous sinus thrombophlebitis and meningitis that was successfully treated with long-term antibiotics with right sphenoidotomy.
Osteopetrosis is a rare bone disease characterized by bones abnormally dense and prone to breakage due to defective osteoclastic function. There are two forms of osteopetrosis based on the osteoclast counts: one is osteopetrosis with normal or increased osteoclast counts, the bone resorption defect usually results from failure to produce a ruffled border,[1] and the other is decreased osteoclast counts, an abnormality in the molecular pathways involved in osteoclastogenesis has been suggested.[2] Johnston et al.[3] distinguished osteopetrosis by their pattern of inheritance: a malignant form seen in childhood and inherited in an autosomal recessive osteopetrosis (ARO), a benign form designated autosomal dominant osteopetrosis (ADO). ADO type I (ADO I) features a generalized, diffuse osteosclerosis affecting especially the cranial vault. Whereas ADO type II (ADO II) is characterized primarily by vertebral endplate thickening ("sandwich vertebra" or "rugger-jersey spine" appearance) which includes increased cortical but normal cancellous bone volume, fragile bones with multiple fractures.[4,5] Here we present a case of ADO II in a 52-years-old female, which was complicated by the development of cavernous sinus thrombophlebitis and meningitis.
A 52-years-old female was visited to our hospital emergency department due to abrupt onset of headache, fever, and vomiting. On her past history she suffered from chronic sinusitis 10 years ago. Two years ago, she received right total maxillectomy for treatment of right maxillary sinus infection with necrosis. Since one year ago, she experienced orthopedic surgery of open reduction and internal fixation of both femur due to slip down injury (Fig. 1). She had no history of hypertension, diabetes, hepatitis, and tuberculosis. Family history of other family members could not obtained except her elder sister because she did not wanted disclose her disease further to her family members. Her elder sister diagnosed osteopetrosis 12 years ago during dental procedure and had past history of femur fracture since 22 years ago. On her social history, she took intermittent alcohol consumption without smoking.
On arrival at the emergency department, she had a systolic blood pressure of 110 mmHg, diastolic pressure of 70 mmHg, pulse rate of 133 per minute, breathing rate of 22 per minute, body temperature of 38.3℃. She had 153 cm of height, 40.1 kg of body weight, and 17.1 kg/m2 of body mass index (BMI). On physical and neurological examination, she showed right eye proptosis and lateral gaze palsy, left hearing disturbance, and left facial nerve area sensory deficit.
Laboratory examinations were performed at the time of visiting; leukocyte was 14,000/mm3 (reference range 4,000-10,000), hemoglobin was 10.6 g/dL (reference range 12.0-16.0), platelet count was 354,000/mm3 (reference range 140,000-400,000), erythrocyte sedimentation rate was 81 mm/hr (reference range 0-20), and C-reactive protein was 18.12 mg/dL (reference range 0-0.50). Blood chemistry showed serum albumin 3.7 g/dL (reference range 3.5-5.0), total calcium 7.8 mg/dL (reference range 7.8-10.0), phosphorus 1.6 mg/dL (reference range 2.9-4.3), ionized calcium 0.89 mM/L (reference range 0.96-1.40), sodium 125 mM/L (reference range 137-150), potassium 4.1 mM/L (reference range 3.5-5.3), chloride 87 mmol/L (reference range 99-110), bicarbonate 28 mmol/L (reference range 22-34).
Radiographs of chest and PNS showed diffuse sclerotic change in bony structures (Fig. 2). Brain magnetic resonance imaging (MRI) showed leptomeningitis with abscess formation along right Meckel's cave and right cavernous sinus wall (Fig. 3A, B).
Empirical parenteral antibiotics were started with ceftriaxone, metronidazole, and vancomycin for cavernous sinus thrombophlebitis and meningitis. Antibiotics were changed to ceftriaxone alone after obtained blood culture results with streptococcus constellatus, milleri infection.
Ten days after admission, she showed symptoms of febrile sensation, vomiting, whirling type vertigo, and generalized tonic chronic seizure attack. Mental status changed from alert to deep drowsy. On brain MRI scan, epidural empyema along right frontotemporal convexity and aggravation of meningeal enhancement were seen (Fig. 3C, D). She was moved to intensive care unit and antibiotics were changed to vancomycin and metronidazole because antibiotics therapy with ceftriaxone alone caused aggravation of patient's symptom. Intravenous (IV) steroid and mannitol were administered to control increased intracranial pressure. Prompt neurosurgical decompression was indicated, but neurosurgery was not done due to refusal of operation by family members, patient's poor nutritional status like extremely low BMI and sclerotic bone disease with possibility of nonunion after operation. On cerebrospinal fluid (CSF) examination, red blood cell 0/mm3, leukocyte 800/mm3 on fluid analysis, but micro-organism was not grown.
Thirteen days after admission, she was transferred to general ward because her mental status changed to alert again with no further seizure attack. Seventeen days after admission, fever was developed again, so metronidazole was changed to meropenem to cover broader spectrum of infection. Vancomycin was stopped for 2 weeks later because fever was developed during previous vancomycin usage, and maintained meropenem alone for further 10 weeks. On endoscopic examination, sphenoid sinus was suspected to focus of infection. Five weeks after admission, she got a right sphenoidotomy under local anesthesia. Complete resolution of infection was obtained after broad spectrum antibiotic therapy for 12 weeks.
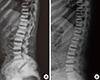
For etiologic evaluation, we tested further examination for diffuse sclerosing bony structure. Laboratory data revealed elevated serum levels of tartrate resistant acid phosphatase (TRAP) without carbonic anhydrase II DNA mutation, the intact parathyroid hormone (PTH) was elevated by 144.04 pg/mL (reference range 15-65), the 1,25-dihydroxy-vitamin D (1,25-[OH]2D) was elevated by 91.0 pg/mL (reference range 20.0-60.0), and the 25-hydroxy-vitamin D3 (25-[OH]D3) was decreased by 11.43 ng/mL (reference range 20-30). Results of bone formation and resorption marker were various with the value of bone alkaline phosphatase isoenzyme 34.2 g/L (age and gender specific reference range below 22), osteocalcin 10.4 ng/mL (age and gender specific reference range 4.0-12.0), and deoxypyridinoline 9.5 nM deoxypyridinoline (DPD)/mM (reference range 3.0-7.4), respectively. A plain radiograph of the spine showed end-plate thickening and sclerosis producing the classic "sandwich vertebra" appearance (Fig. 4A). Bone mineral densitometry showed increased lumbar spine vertebral bone density and bone mineral content values (bone mineral density 2.713 g/cm2 with T-score +14.84, bone mineral content of lumbar spine 173.28 g). For evaluation of chloride channel 7 (CLCN7) deficiency osteopetrosis, we performed a CLCN7 gene mutation analysis using genomic DNA which was extracted from peripheral blood using genomic DNA prep kit (Solgent Co. Ltd., Daejeon, Korea). But we could not found any mutations on 24 and 25 exon of CLCN7 gene. And her older sister's spine X-ray also showed sandwich vertebra appearance (Fig. 4B) and the CLCN7 gene mutation was not found, too.
Osteopetrosis is a rare hereditary bone disorder due to defective osteoclastic function. Among several types of osteopetrosis, our patient was corresponded to ADO II because she showed typical radiographic findings of characteristic vertebral endplate thickening of Albers-Schönberg disease. ADO II was described in 1904 by Albers-Schönberg[6] and usually diagnosed by the discovery of typical skeletal changes in young adults who undergo radiologic evaluation of a fracture. The prevalence is 1 in 100,000 to 1 in 500,000 adults.[7] It findings may include fractures in any long bone and/or the posterior arch of a vertebra and scoliosis and hip osteoarthritis, osteomyelitis of the mandible or septic osteitis or osteoarthritis elsewhere.[8]
Increases in the serum concentrations of the TRAP and the creatine kinase isoenzyme BB (CKBB) have been reported as biologic markers of ADO II. It could reflect increased osteoclast numbers and could help identify affected individual who do not have diagnostic radiographic findings.[9,10] Increased serum concentration of TRAP, recurrent infection and increased vertebral bone mass would identify this form as ADO II and these findings were observed also in our patient.
Mutations in the CLCN7 gene that encodes an osteoclast-specific chloride channel are well known cause of ADO II. And the disease most probably described by Albers-Schönberg[6] turned out to be a form of Chloride Channel 7 Deficiency Osteopetrosis.[11] However, it has to be noted that a mutation in CLCN7 has not been demonstrated in up to 30% of patients presenting with a clinical phenotype of ADO, indicating further heterogeneity.[1,10] Based on the preponderance of missense mutations and the knowledge that chloride channels probably function as dimers, it seems that heterozygous CLCN7 gene mutations may cause ADO II through a dominant negative mechanism.[12] In our patient, we could not found any mutations on 24 and 25 exon of CLCN7 gene.
Serum calcium may be low in severe disease, and PTH and 1,25-(OH)2D levels may be elevated in response to hypocalcemia.[7] Our patient also showed low serum calcium level and elevated PTH and 1,25-(OH)2D and these suggest our patient has severe disease. Closure of bone foramina causes cranial nerve compression with visual and hearing deterioration in osteopetrosis patients as shown in our patient.[1]
Diagnosis of cavernous sinus thrombosis is usually made with MRI scan with venogram. The CSF culture may be positive if there is coexisting meningitis. Duration of parenteral antimicrobial therapy should be at least 4 weeks.[13] Cavernous sinus thrombosis is the formation of a blood clot within the cavernous sinus and is caused usually from a spreading infection in the nose, sinuses, or teeth. Cavernous sinus thrombosis symptoms include: decrease or loss of vision, chemosis, exophthalmos, headaches, and paralysis of the cranial nerves that course through the cavernous sinus,[14] and some features such as exophthalmos and headaches were observed in our patient.
This infection is life-threatening and requires immediate treatment, which usually includes antibiotics and sometimes surgical drainage. Prior chronic sinusitis and osteomyelitis are important risk factors. Therefore, the diagnosis of septic cavernous sinus thrombosis requires a high index of suspicion and confirmation by imaging like MRI scan with venogram or contrast enhanced computed tomography (CT) scan; early diagnosis and surgical drainage of the underlying primary source of infection in conjunction with long-term IV antibiotic therapy are critical for an optimal clinical outcome.[15] Cure was achieved in our patient with aggressive management including long term antibiotics with right sphenoidotomy.
Empirical antimicrobial therapy is initiated in patients with suspected bacterial meningitis before the results of CSF Gram's stain and culture are known. Usually empirical antimicrobial therapy consists of a third- or fourth-generation cephalosporin and vancomycin, and metronidazole is added to the empirical regimen to cover gram-negative anaerobes in patients with otitis, sinusitis, or mastoiditis.[16] Our patient was suspected bacterial meningitis with sinusitis, so initial empirical antimicrobial agents were combination of ceftriaxone, vancomycin and metronidazole.
Our patient showed hyponatremia and hypochloremia on admission. Most possible cause of hyponatremia in our patient was syndrome of inappropriate antidiuretic hormone (SIADH) because central nervous system (CNS) infection is common cause of SIADH, low plasma osmolality (274 mOsm/kg) and inappropriate urinary concentration (urine osmolality 526 mOsm/kg) with hyponatremia was corrected after control of infection. Possible causes of hypochloremia in our patient were metabolic alkalosis due to vomiting and combined with hyponatremia because initial presenting symptom included vomiting and hypochloremia was resolved by correction of hyponatremia.
Unfortunately patient did not visit after discharge, so we could not obtain bone marker follow up data. But decreased level of ionized calcium was recovered and intact PTH was decreasing after stabilization of patient's status.
Here we have presented the first case of cavernous sinus thrombophlebitis and meningitis from chronic osteomyelitis in patient with osteopetrosis. The treatment methods of meningitis could be different in patients with sclerotic bone disease because they had higher possibility of nonunion after operation. Early find and removal of suspected focus of infection and usage of long-term susceptible antibiotics are mandatory to control of infectious disease especially in patient with osteopetrosis.
Figures and Tables
Fig. 1
Radiograph showed that internal fixation state of both femurs due to shaft fracture of both femurs, a common complication of autosomal dominant osteopetrosis type II.
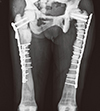
Fig. 2
Radiographs of chest (A) and peripheral nervous system (PNS) (B) showed diffuse sclerotic change in whole bony structures. Both ethmoid and maxillary sinusitis with postoperative state of right maxillary sinus was seen.
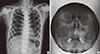
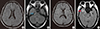
Fig. 3
Brain magnetic resonance imaging (MRI) showed that abnormal leptomeningeal enhancement along both hemisphere, more pronounced right side than left side (A) and abscess formation with abnormal enhancement along right Meckel's cave (blank arrow), right cavernous sinus wall, right side prepontine and mesencephalic cistern, and choroid fissure (B). At hospital day 10, follow up brain MRI showed that aggravation of meningeal enhancement (C) and epidural empyema along right frontotemporal convexity and brain abscess with peripheral edema (filled arrow) in right anterior temporal lobe (D).
References
1. Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008; 42:19–29.

2. Sobacchi C, Frattini A, Guerrini MM, et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet. 2007; 39:960–962.

3. Johnston CC Jr, Lavy N, Lord T, et al. Osteopetrosis. A clinical, genetic, metabolic, and morphologic study of the dominantly inherited, benign form. Medicine (Baltimore). 1968; 47:149–167.
4. Bollerslev J, Andersen PE Jr. Radiological, biochemical and hereditary evidence of two types of autosomal dominant osteopetrosis. Bone. 1988; 9:7–13.

5. Bénichou OD, Laredo JD, de Vernejoul MC. Type II autosomal dominant osteopetrosis (Albers-Schonberg disease): clinical and radiological manifestations in 42 patients. Bone. 2000; 26:87–93.

6. Albers-Schönberg HE. Röntgenbilder einer seltenen knochenerkrankung. Munch Med Wochenschr. 1904; 51:365–368.
7. Favus MJ, Vokes TJ. Chapter 355. Paget's disease and other dysplasias of bone. In : Longo DL, Fauci AS, Kasper DL, editors. Harrison's principles of internal medicine. 18th ed. New York, NY: McGraw-Hill;2012.
8. de Vernejoul MC, Schulz A, Kornak U. CLCN7-related osteopetrosis. 2007. cited by 2013 Jun 20. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1127/.
9. Waguespack SG, Hui SL, White KE, et al. Measurement of tartrate-resistant acid phosphatase and the brain isoenzyme of creatine kinase accurately diagnoses type II autosomal dominant osteopetrosis but does not identify gene carriers. J Clin Endocrinol Metab. 2002; 87:2212–2217.

10. Del Fattore A, Peruzzi B, Rucci N, et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J Med Genet. 2006; 43:315–325.

11. Whyte MP, Kempa LG, McAlister WH, et al. Elevated serum lactate dehydrogenase isoenzymes and aspartate transaminase distinguish Albers-Schonberg disease (Chloride Channel 7 Deficiency Osteopetrosis) among the sclerosing bone disorders. J Bone Miner Res. 2010; 25:2515–2526.

12. Campos-Xavier AB, Saraiva JM, Ribeiro LM, et al. Chloride channel 7 (CLCN7) gene mutations in intermediate autosomal recessive osteopetrosis. Hum Genet. 2003; 112:186–189.

13. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's principles and practices of infectious diseases. 7th ed. Philadelphia, PA: Elsevier Churchill Livingstone;2005.
14. Clarke M, Enuh H, Saverimuttu J, et al. Streptococcus group C meningitis with cavernous sinus thrombosis. Infect Drug Resist. 2013; 6:79–81.
15. Cannon ML, Antonio BL, McCloskey JJ, et al. Cavernous sinus thrombosis complicating sinusitis. Pediatr Crit Care Med. 2004; 5:86–88.

16. Roos KL, Tyler KL. Chapter 381. Meningitis, encephalitis, brain abscess, and empyema. In : Longo DL, Fauci AS, Kasper DL, editors. Harrison's principles of internal medicine. 18th ed. New York, NY: McGraw-Hill;2012.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download