Abstract
After discontinuation of bisphosphonate therapy, an antiresorptive effect and antifracture protection persist for an undefined period. Patients are encouraged to continue calcium and vitamin D supplementation, during a bisphosphonate drug holiday. However, assessment of adequate calcium intake during the bisphosphonate drug holiday is difficult. Therefore, we measured the serum intact parathyroid hormone (PTH) level as a surrogate marker. A premenopausal woman discontinued bisphosphonate therapy, after 7.5 years of treatment. Two months later, blood calcium and phosphorus levels were normal, serum 25-hydroxyvitamin D level was 31.3 ng/mL, but serum PTH level had increased to 94.9 pg/mL. The elemental calcium supplement dose was increased to 600 mg/day, with no change in the cholecalciferol dose (400 IU). Her serum PTH levels decreased to 49.1 after 4 months and 32.9 pg/mL after 5 months. The serum PTH level may be helpful in assessing adequate calcium intake during a bisphosphonate drug holiday.
Osteoporosis is a skeletal disorder characterized by compromised bone strength (reflecting a combination of bone density and bone quality), predisposing an individual to an increased risk of fracture (National Institutes Consensus Development Conference in March 2000).[1] Bisphosphonates have been widely used as first-line therapy with proven antifracture efficacy in patients with osteoporosis, reducing the risk of fracture at the hip, vertebrae, and other nonvertebral sites.[2,3] Bisphosphonates bind to hydroxyapatite crystals in bone, particularly at sites of active bone remodeling, and decrease bone resorption by inhibiting osteoclast function, with a consequent increase in bone mass, mainly due to refilling of the remodeling space and an increase in bone mineralization.[4,5,6] However, bisphosphonate therapy has been associated with adverse events such as acute-phase reaction, hypocalcemia, esophageal disease, atrial arrhythmia, osteonecrosis of the jaw, atypical femur fracture, and severe suppression of bone turnover.[2,5,6] The effects of most therapies resolve immediately after drug discontinuation, but those of bisphosphonates do not. If the adverse effects of these drugs are associated with the long residence time in bone, resolution of these adverse effects may take a long time.[2] Bisphosphonates accumulated in bone after years of treatment are gradually released over years,[2,5,6] and therefore, discontinuation of bisphosphonate therapy is associated with an antiresorptive effect and antifracture protection persisting for an undefined period, and results in a minimization of the potential risks of adverse events.[2,7]
Ott[8] recommends that patients who discontinue bisphosphonate treatment are encouraged to continue calcium and vitamin D supplementation, although there are no clinical data on how patients should be managed and monitored during a bisphosphonate drug holiday. Calcium and vitamin D supplements are known to be beneficial for bone health.[9] Recently, however, the cardiovascular safety of calcium supplements has been questioned,[10,11,12] and calcium supplementation may be inadequate in some patients during the bisphosphonate drug holiday. Assessment of adequate calcium intake from dietary and/or supplemental sources is difficult, especially when the serum vitamin D level is neither insufficient nor deficient. Therefore, we suggest that the serum intact parathyroid hormone (PTH) level may help in assessing the achievement of adequate calcium intake, because some of the bound bisphosphonate is released from bone and is metabolically active, [2] causing calcium efflux inhibition,[13] and increasing serum PTH level in cases of insufficient calcium supplementation during a bisphosphonate drug holiday.
In the case reported here, calcium supplementation during the bisphosphonate drug holiday was not adequate. The patient's serum PTH level increased, although the serum calcium and 25-hydroxyvitamin D (25-[OH]D) levels were within the normal range during the bisphosphonate drug holiday. Further to our recommendation, the dose of calcium supplements was increased, and the patient's serum PTH level normalized.
The 37-year-old Korean woman (height, 161.5; weight, 51.5 kg; body mass index, 19.7 kg/m2) was referred from a health promotion center for low bone mineral density (BMD). She was normally menstruating, premenopausal, and was a housewife. She had no history of pregnancy, breastfeeding, oligo- or amenorrhea, or other history consistent with premenopausal estrogen deficiency, fractures, or kidney stones. She did not take any medication. She did not exercise on a regular basis. Regarding family history, her mother had gastric cancer and osteoporosis but no history of fracture. There were no symptoms or signs of secondary causes of osteoporosis such as Cushing's syndrome, thyrotoxicosis, celiac disease, malabsorption, early menopause, renal or liver disease, rheumatoid arthritis, systemic lupus erythematosus, or other inflammatory conditions, or connective tissue disorders during the physical examination.
The laboratory evaluation aimed to identify secondary causes of osteoporosis. Laboratory blood test results were as follows: leukocyte count, 4,030/µL; hemoglobin level, 12.8 g/dL; mean corpuscular volume, 88.2 fL; aspartate aminotransferase level, 11 IU/L (normal range, 0-40 IU/L); alanine aminotransferase level, 20 IU/L (normal range, 0-40 IU/L); γ-gamma-glutamyl transpeptidase level, 17 IU/L (normal range, 11-50 IU/L); alkaline phosphatase level, 65 IU/L (normal range, 38-126 IU/L); total protein level, 7.0 g/L (normal range, 6-8 g/L); albumin level, 4.0 g/L (normal range, 3.1-5.2 g/L); uric acid level, 4.1 mg/dL (normal range, 3-7 mg/dL); creatinine level, 0.7 mg/dL (normal range, 0.5-1.1 mg/dL); fasting blood glucose level, 75 mg/dL; total cholesterol level, 88 mg/dL; triglyceride level, 75 mg/dL; low density lipoprotein-cholesterol level, 79 mg/dL; high density lipoprotein-cholesterol level, 40 mg/dL; sodium level, 140 mEq/L (normal range, 136-142 mEq/L); potassium level, 3.9 mEq/L (normal range, 3.5-5.3 mEq/L); calcium level, 8.4 mg/dL (normal range, 8.2-10.8 mg/dL); phosphorus level, 2.8 mg/dL (normal range, 2.5-4.5 mg/dL); and intact PTH level, 26.1 pg/mL (normal range, 13-54 pg/mL). With regard to bone turnover markers, the serum osteocalcin level was 14.5 ng/mL (normal range, 4-12 ng/mL) and the urinary free deoxypyridinoline/creatinine ratio was 8.0 nmol/mmol (normal range, 3.0-7.4 nmol/mmol). In thyroid function tests, the free T4 level was 13.3 pmol/L (normal range, 10.0-28.2 pmol/L) and the thyroid stimulating hormone level was 2.2 mIU/L (normal range, 0.5-4.7 mIU/L). The serum estradiol level was 97.4 pg/mL (normal range, 18.9-570.8 pg/mL in normally menstruating females) and the follicle stimulating hormone level was 3.3 IU/L (normal range, 1.5-33.4 IU/L in normally menstruating females). Urinalysis findings were normal. Findings of radiologic evaluations, including abdominal, pelvic, and thyroid ultrasonography and chest radiography, were nonspecific. Dual energy X-ray absorptiometry (DXA) Norland (Norland Corp., Fort Atkinson, WI, USA) was used to measure BMD in the lumbar vertebrae L2-L4 (BMD 0.635 g/cm2, T-score -4.3, Z-score -3.6) and femoral neck (BMD 0.634 g/cm2, T-score -2.4, Z-score -2.4).
The patient had no evidence of other medical diseases or secondary osteoporosis as determined by physical examination and laboratory evaluation. The patient with a BMD Z-score -3.6 of the lumbar vertebrae L2-L4 and a Z-score -2.4 of femoral neck was categorized as having BMD that is "below expected range for age" in premenopausal woman. The patient's condition was diagnosed as idiopathic osteoporosis. We prescribed 10 mg alendronate, 600 mg elemental calcium, and 400 IU cholecalciferol (vitamin D3) daily to treat as well as prevent further progression of the osteoporosis, because she did not want to get pregnant and her BMD Z-score for the lumbar vertebrae L2-L4 was -3.6. The treatment was continued for 4 years and 6 months and then changed to a combination of alendronate (70 mg) and cholecalciferol (5,600 IU) (Fosamax Plus D 70 mg) once weekly for 3 years. Therefore, the total duration of bisphosphonate therapy was 7 years and 6 months. Evaluations such as physical examination, regular BMD measurement, blood biochemistry tests, bone turnover marker measurement, lipid profile analysis, urinalysis, and radiologic studies were performed during treatment.
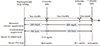
At 7 years and 6 months of bisphosphonate treatment, BMD was measured using Lunar iDXA (GE Healthcare Lunar, Madison, WI, USA). BMD in the lumbar vertebrae L2-L4 was 0.763 g/cm2 (T-score -3.4, Z-score -3.0) and that in the femoral neck was 0.739 g/cm2 (T-score -1.7, Z-score -1.3) (Table 1). After treatment, the percentage change in BMD was 20% for L2-L4 and 17% for the femoral neck (Table 1). The serum level of the bone turnover marker C-terminal telopeptides type I collagen was 0.17 ng/mL (normal range, 0.01-0.60 ng/mL in premenopausal women) before the start of the drug holiday (baseline). We discussed the details of a bisphosphonate drug holiday with the patient. A drug holiday was recommended because the T-score was -1.7 and the Z-score was -1.3 for the femoral neck after 7 years and 6 months of bisphosphonate treatment and because there was no fracture during bisphosphonate (alendronate) therapy. The patient decided to take a bisphosphonate drug holiday. She started to take a multivitamin including 100 mg elemental calcium and 400 IU cholecalciferol without bisphosphonate. Two months later, her calcium level was 8.7 mg/dL; phosphorus level, 2.8 mg/dL; and serum PTH level, 94.9 pg/mL. Because of the increased PTH level, we measured the serum 25-(OH)D level, which was 31.3 ng/mL, and 24-hour calcium excretion, which was 171.4 mg (normal range, 100-300 mg). The serum PTH level was measured using the ADVIA Centaur XP Immunoassay System (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). The serum 25-(OH)D level was measured using chemiluminescent immunoassay LIAISON® 25-(OH)D TOTAL Assay kit (DiaSorin Inc., Stillwater, MN, USA) on a LIAISON® automatic analyzer (DiaSorin Inc.). Because the serum PTH level had increased to 94.9 pg/mL, although the patient did not show a vitamin D deficiency (serum 25-[OH]D level, 31.3 ng/mL), we recommended increasing the amount of supplemental calcium to 600 mg/day elemental calcium (1,500 mg/day as calcium carbonate) with no change in the cholecalciferol dose (400 IU). Dairy product intake or calcium intake from dietary sources assessed by dietitian were not changed before, during bisphosphonate treatment, or after bisphosphonate discontinuation. Four months later, the patient's serum calcium level was 8.6 mg/dL; phosphorus level, 2.3 mg/dL; and PTH level, 49.1 pg/mL. Five months later, her serum calcium level was 8.9 mg/dL; phosphorus level, 2.8 mg/dL; and PTH level, 32.9 pg/mL (completely normalized, Fig. 1). She is currently being followed up without complications.
Whitaker et al.[14] suggested that the decision regarding a bisphosphonate drug holiday in patients with osteoporosis must be based on patient preference and on individual assessment of the potential benefits and risks, to optimize the efficacy of bisphosphonate treatment. These suggestions were based on 3 long-term extension clinical trials (treatment duration, 6-10 years): the Fosamax Fracture Intervention Trial Long-Term Extension (FLEX), the Reclast Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly-Pivotal Fracture Trial (HORIZON-PFT) extension, and the Actonel Vertebral Efficacy with Risedronate Therapy-Multinational Trial (VERT-MN) extension. According to Black et al.,[15] patients with low BMD at the femoral neck and a T-score of less than -2.5 after 3-5 years of treatment and patients with an existing vertebral fracture who have a T-score not higher than -2.0 may benefit from continued bisphosphonate treatment, whereas those with a femoral neck T-score above -2.0 and a low risk of vertebral fracture are unlikely to benefit from such continued therapy. However, until now, there have been no suggestions or studies about a bisphosphonate drug holiday in premenopausal women. In our case, after 7.5 years of bisphosphonate (alendronate) therapy, the femoral neck T-score was -1.7 and the femoral neck Z-score was -1.3; further, no fracture occurred during bisphosphonate (alendronate) therapy. We discussed the bisphosphonate drug holiday with the patient, who decided to discontinue bisphosphonate treatment.
To date, there has been no guidance from clinical trials on how patients should be managed and monitored during a bisphosphonate drug holiday or when therapy should be restarted after the bisphosphonate drug holiday. McClung et al.[2] suggested that measuring BMD and biochemical bone turnover marker levels 2-3 years after bisphosphonate discontinuation may provide information about the persistence of the effect of the retained bisphosphonate in bone. Ott[8] recommends that patients who discontinue bisphosphonate be encouraged to continue calcium and vitamin D supplementation. Physicians can assess the vitamin D nutritional status by measuring the serum 25-(OH)D level. However, assessment of adequate calcium intake from dietary and/or supplemental sources is difficult, especially when the serum vitamin D level is neither insufficient nor deficient.
Sunlight and diet are the 2 major natural sources of vitamin D, although sunlight is the major contributor, as normal diets have low vitamin D content.[16] Many individuals aged >50 years take vitamin D supplements, and the average vitamin D intake is approximately 400 IU daily.[16,17] During the bisphosphonate drug holiday, our patient took 400 IU/day vitamin D (as cholecalciferol). Vitamin D deficiency, insufficiency, and sufficiency are defined by the level of serum 25-(OH)D.[16] Vitamin D deficiency is characterized by intestinal malabsorption of calcium and phosphorus, hypocalcemia, low 1,25-(OH)D levels, secondary hyperparathyroidism, and demineralization of bone.[16] In 2011, the Institute of Medicine defined vitamin D insufficiency as a level of <20 ng/mL,[17] and The Endocrine Society defined it as a level of 20-29 ng/mL,[18] but the serum levels of 25-(OH)D that define vitamin D insufficiency and deficiency have not been established.[16] The serum 25-(OH)D threshold is 5-10 ng/mL for normal calcium absorption.[19] The serum PTH level is inversely related to the serum 25-(OH)D level; with an increase in the serum 25-(OH)D level, there is a decrease in the serum PTH level that usually reaches a plateau.[16,20,21] The serum 25-(OH)D level at which the serum PTH levels plateaus varies from 12 to 50 ng/mL (with most values being <30 ng/mL).[16,20] During the bisphosphonate drug holiday, the serum 25-(OH)D level of our patient was 31.3 ng/mL and 24-hour calcium excretion was 171.4 mg (normal range, 100-300 mg), with an increase in the serum PTH level to 94.9 pg/mL (normal range, 13-54 pg/mL). Our patient had neither urinary calcium loss nor vitamin D insufficiency or deficiency.
For decades, the use of calcium supplements in healthy adults has been a consistent public health message.[22] When calcium intake from dietary and/or supplemental sources is insufficient, compensatory loss of calcium from the bone occurs.[23] The calcium concentration in extracellular fluid is tightly maintained within a narrow range.[23] The bone, the gut, and the kidney play a major role in calcium homeostasis.[23] The bone is the major calcium reservoir in the body.[23] Ordinarily, as a result of normal bone turnover, approximately 500 mg of calcium per day is released from bone and an equivalent amount is accreted to bone daily.[23] Because bisphosphonates are potent inhibitors of osteoclastic bone resorption, such inhibition reduces calcium efflux from the skeleton, which is normally followed by transient slight hypocalcemia during bisphosphonate drug therapy.[13] Most patients do not become hypocalcemic because of the compensatory increase in PTH secretion during bisphosphonate therapy.[13] Bisphosphonates require adequate calcium intake to deposit new bone mineral.[24] Calcium supplements or combination calcium/vitamin D supplements are recommended during bisphosphonate therapy to maximize the efficacy of bisphosphonate therapy.[24,25] It is reasonable then that patients take calcium and vitamin D supplements during the bisphosphonate drug holiday, during which period the effect of the retained bisphosphonate in bone might persist.[2] However, calcium supplements have been reported to increase the risk of cardiovascular events and stroke,[10,11,12,26] although a causal relationship between calcium supplementation and cardiovascular events remains inconsistent and inconclusive.[22] It is now generally recommended that our calcium requirement should be obtained from dietary source, rather than from supplemental source.[26,27,28] Some patients, therefore, do not take enough calcium supplements during the bisphosphonate drug holiday. Our patient also did not take enough calcium supplements. It is difficult for clinicians to assess whether patients achieve adequate calcium intake from dietary and/or supplemental sources during a bisphosphonate drug holiday, especially when the serum vitamin D level is neither insufficient nor deficient, as was the case in our patient. We postulated that in this situation, the serum PTH level may be helpful in assessing whether a patient had achieved an adequate calcium intake. After discontinuing bisphosphonate treatment, our patient started taking a multivitamin supplement containing 100 mg elemental calcium and 400 IU cholecalciferol. Two months later, the patient's blood calcium and phosphorus levels were normal, and serum PTH level had increased to 94.9 pg/mL. At this time, the patient's serum 25-(OH)D level was 31.3 ng/mL; therefore, the elemental calcium dose was increased to 600 mg with no change in the cholecalciferol dose (400 IU). Four months later, the serum PTH level was 49.1 pg/mL, and 5 months later, this level had completely normalized to 32.9 pg/mL. We believe that our case report is of considerable interest to the medical community dedicated to understanding the relationships between the serum PTH level, calcium supplementation, and the sustained effect of the retained bisphosphonate in bone during a bisphosphonate drug holiday. This study had one limitation in that we measured BMD initially using DXA Norland but BMD at the follow-up was measured using Lunar iDXA. In conclusion, adequate calcium and vitamin D supplementation is important for patients who cannot achieve an adequate dietary calcium intake after bisphosphonate discontinuation, and the serum PTH level may be helpful in assessing whether a patient has achieved adequate calcium intake during a bisphosphonate drug holiday.
Figures and Tables
Fig. 1
Serum parathyroid hormone levels during the bisphosphonate drug holiday. This scheme shows the serum parathyroid hormone levels of the patient who was taking elemental calcium supplements, 2 months, 6 months, and 7 months after the start of the bisphosphonate drug holiday. 25-(OH)D, 25-hydroxyvitamin D; PTH, intact parathyroid hormone.
References
1. Kleerekoper M. Osteoporosis overview. In : Rosen CJ, Bouillon R, Compston JE, editors. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. New York, NY: Wiley-Blackwell;2013. p. 345–347.
2. McClung M, Harris ST, Miller PD, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013; 126:13–20.

3. MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008; 148:197–213.

4. Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008; 19:733–759.

5. Khosla S, Bilezikian JP, Dempster DW, et al. Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab. 2012; 97:2272–2282.

6. Diab DL, Watts NB. Bisphosphonates in the treatment of osteoporosis. Endocrinol Metab Clin North Am. 2012; 41:487–506.

8. Ott SM. What is the optimal duration of bisphosphonate therapy? Cleve Clin J Med. 2011; 78:619–630.

9. Prentice A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004; 7:227–243.

10. Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008; 336:262–266.

11. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010; 341:c3691.

12. Bolland MJ, Grey A, Avenell A, et al. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ. 2011; 342:d2040.

13. Peter R, Mishra V, Fraser WD. Severe hypocalcaemia after being given intravenous bisphosphonate. BMJ. 2004; 328:335–336.

14. Whitaker M, Guo J, Kehoe T, et al. Bisphosphonates for osteoporosis-where do we go from here? N Engl J Med. 2012; 366:2048–2051.

15. Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis-for whom and for how long? N Engl J Med. 2012; 366:2051–2053.

16. Gallagher JC. Vitamin D insufficiency and deficiency. In : Rosen CJ, Bouillon R, Compston JE, editors. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. New York, NY: Wiley-Blackwell;2013. p. 624–631.
17. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011; 96:53–58.

18. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–1930.

19. Need AG, O'Loughlin PD, Morris HA, et al. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res. 2008; 23:1859–1863.

20. Sai AJ, Walters RW, Fang X, et al. Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab. 2011; 96:E436–E446.

21. Sahota O, Mundey MK, San P, et al. The relationship between vitamin D and parathyroid hormone: calcium homeostasis, bone turnover, and bone mineral density in postmenopausal women with established osteoporosis. Bone. 2004; 35:312–319.

23. Favus MJ, Goltzman D. Regulation of calcium and magnesium. In : Rosen CJ, Bouillon R, Compston JE, editors. Primer on the metabolic bone diseases and disorders of mineral metabolism. 8th ed. New York, NY: Wiley-Blackwell;2013. p. 173–179.
24. Rush DN, Jones MW, Futrell DP, et al. Evaluation of calcium and vitamin D supplementation in bisphosphonate therapy. J Am Pharm Assoc (2003). 2007; 47:725–728.

25. Ringe JD, van der Geest SA, Möller G. Importance of calcium co-medication in bisphosphonate therapy of osteoporosis: an approach to improving correct intake and drug adherence. Drugs Aging. 2006; 23:569–578.

26. Reid IR. Should we prescribe calcium supplements for osteoporosis prevention? J Bone Metab. 2014; 21:21–28.

27. Sugerman DT. JAMA patient page. Osteoporosis. JAMA. 2014; 311:104.
28. Manson JE, Bassuk SS. Calcium supplements: do they help or harm? Menopause. 2014; 21:106–108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download