Abstract
Numerous causes of hypertrophic osteoarthropathy (HOA) have been reported. Commonly, secondary osteoarthropathy accompanies pulmonary diseases such as carcinoma of the lung, pleural tumors, lung abscesses, and bronchiectasis. However, HOA in inflammatory bowel disease is a rare complication. There are only a few reports of secondary HOA with Crohn's disease. Our purpose was to report another case of HOA in Crohn's disease. We describe a case of a 27-year-old man with underlying Crohn's disease presenting with 2 years of pain in multiple joints. Radiographic findings suggested HOA in extremities. We performed a conservative treatment including medication and rehabilitations. The patient's symptoms were much improved at the latest follow-up. Although numerous studies on HOA have been published, the pathogenesis of HOA is still unclear. Various treatment modalities were recommended but further studies to uncover the pathogenesis of HOA with Crohn's disease and to establish a treatment modality are needed.
Primary hypertrophic osteoarthropathy (HOA, pachydermoperiostosis) is a rare hereditary disease without any underlying disease, which accompanies hypertrophy of the digits, arthritis/arthralgia, and periosteal new bone formation.[1] Some patients complain of hypertrophic skin changes (pachyderma) and hyperhidrosis as well.[2]
Also, secondary HOA (Pierre Marie-Bamberger syndrome), which was first observed by Von Bamburger in 1889 and Marie in 1890, is well known to have similar symptoms to primary HOA in patients with underlying diseases such as pulmonary diseases mostly.[1,3] However, HOA in patients with inflammatory bowel disease such as Crohn's disease and ulcerative colitis have very rarely been reported.[2]
Recently, we treated secondary HOA in a young man with inflammatory bowel disease. We report the radiological findings, clinical manifestation, and treatment, with literature reviews.
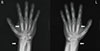
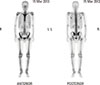
A 27-year-old man with hyperhidrosis on both his palm and sole and digital clubbing presented with a 2-year history of multiple joint pain. He described his symptoms as "becoming stiffer in the joints". In his past medical history, 6 years prior, he had first visited the out-patient department of the Gastroenterology for dyspepsia. Esophagogastroduodenoscopy and a single contrast barium study for the colon had been done. He was diagnosed with Crohn's disease. He had no other endocrinologic, pulmonary or cardiac problems. He had no family history of HOA. In physical examinations, his range of motion was full. Radiographs of both upper and lower extremities showed symmetric cortical thickening and extensive periosteal reactions (Fig. 1A, B, 2). Mild soft tissue swelling in distal portion of both fingers possibly represents clubbing fingers (Fig. 2). There was no evidence of joint space narrowing. He had no sacroiliitis or regional osteoporotic change of the axial bone in X-ray. A nuclear medicine 99mTc hydroxymethane diphosphonate (99mTc-HDP) bone scan showing bilateral symmetrical cortical uptake in both femurs, tibias and fibulas suggested HOA (Fig. 3). Laboratory findings were negative for rheumatoid factor, human leukocyte antigen (HLA) B27, and the antinuclear antibodies. The erythrocyte sedimentation rate was elevated by 53 (normal range, 0-10) mm/h, and the high-sensitivity C-reactive protein was elevated by 28.2 (normal range, 0-1.0) mg/L as well. He was prescribed celecoxib (Celebrex®, Pfizer Inc., New York, USA) for pain control and showed significant improvement of pain in both limbs at the latest follow-up.
The patient presented with multiple joint pain and tubular bone hypertrophic osteopathies. He had Crohn's disease with digital clubbing, arthralgia, periosteal new bone formation, and hyperhidrosis, which are similar to secondary hypertrophic pulmonary osteoarthropathy. To our knowledge, this is the second such case report in the orthopaedic field.
Numerous articles of extra-intestinal manifestations of Crohn's disease such as erythema nodosum, arthritis or skin ulcer were reported, however there are many other extra-intestinal manifestations which are not familiar with surgeons such as cases of the vulva involvement by enteric fistula or the secondary HOA as in our case.[4] Although the association of HOA with inflammatory bowel disease was first documented by Teleky in 1897, the prevalence and pathophysiology of HOA in Crohn's disease is still unknown.[1,5] As a possible mechanism in secondary hypertrophic osteoarthopathy, firstly, the elevated level of prostaglandin E2 (PGE2) in patients may cause HOA. Kozak et al.[6] documented the relation of elevated level of cyclo-oxygenase-2 (COX-2) derived PGE2 and the urinary PGE2 metabolite (PGE-M) with HOA pathogenesis. In addition, numerous documents report that COX-2 is induced by inflammation and malignancy, and that Crohn's disease had significantly increased levels of PGE2 and urine PGE-M compared to the normal group.[7] The increased levels of circulating PGE2 as well as urinary excretion of PGE-M by the patients of HOA, strongly indicate that an increased level of PGE2 is closely related to the pathogenesis of secondary HOA.[6,7] Secondly, Dickenson and Martin[8] hypothesized that due to the pathologic shunting around the pulmonary vasculature by pulmonary disease, many circulating factors such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and platelets which are normally inactivated by the lungs directly enter the systemic vasculature. The platelet clumps precipitate in the distal vasculature and release PDGF. The PDGF brings fibroblast proliferation with increased vascularity and permeability and consequently results in clubbing of the digits.[9] Another factor that may be responsible for the symptoms of HOA is VEGF, which is also derived and induced in hypoxic condition from platelets. VEGF has a function in angiogenesis and osteoblastic differentiation.[9] It has been shown to induce vascular hyperplasia, new bone formation, and edema, which correlates with symptoms of HOA.[1,9] However, despite many years of clinical diagnosis of HOA, the pathogenesis of periostitis and HOA is still unclear. Also, among the many documents on the pathogenesis of HOA with primary lung disease, there is no report proving the pathogenesis of HOA with inflammatory bowel disease.
The treatment option in secondary HOA patients is removing the primary cause or symptomatic treatment by medication. In our case, no surgical treatment was done. There are several therapeutic means for symptomatic care.
In 2006, Kozak et al.[6] addressed the possibility of COX-2-derived PGE2 resulting in HOA pathogenesis. In his study, a 65-year-old woman with recurrent non-small cell lung cancer and adrenal metastasis had clinical symptom improvement of HOA with rofecoxib, a COX-2 inhibitor that has now been withdrawn over safety concerns.
In 2005, Angel-Moreno Maroto et al.[10] reported a patient who had a bypass procedure to treat Fallot's tetralogy with pulmonary artery atresia, and had complete pain relief of HOA symptoms due to octreotide. This probably occurs because the inhibitory effect on the production of VEGF and endothelial proliferation may result in pain relief of HOA.[3,10]
Recently, the use of bisphosphonate which is an inhibitor of osteoclastic bone resorption, seems to take the center stage as a treatment of HOA. In 2011, Jayakar et al.[11] reported a case of a patient with HOA and sarcoidosis who had undergone lung transplant and was treated with zoledronic acid, showing complete pain resolution. Other studies on the use of bisphosphonates in HOA were reviewed, including pamidronate, zoledronic acid, and risedronate. These bisphosphonates have been shown to decrease the level of plasma VEGF in patients.[11] In addition, Kozak et al.[6] assumed that VEGF monoclonal antibodies such as bevacizumab might help the healing of HOA due to its pathogenesis, which is related to VEGF elevation.
Although the first clinical description of secondary HOA was over 100 years ago, and numerous studies on HOA have been published, the pathogenesis of HOA is still obscure. HOA-associated musculoskeletal pain could ravage the patient's body with sickness and could be disabling to patients and to their caretakers. Unfortunately, it is difficult to make a standard therapeutic modality for HOA due to the rarity of the condition and the distinct characteristics of each underlying disease, which limits medication usage. Further studies including multicentric randomized control trials should be carried out, and more efforts to uncover the pathogenesis of HOA with Crohn's disease and establish a treatment modality are needed.
Figures and Tables
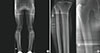 | Fig. 1Entire bone series X-ray (A) and fine focus magnification of the tibia and femur (B) show thick and wavy periosteal reaction involving metaphysis and diaphysis of long bones including both femurs, tibias and fibulas, sparing the epiphysis. Also seen is multilayered periostitis in both tibias which suggests disease progression. |
References
1. Nguyen S, Hojjati M. Review of current therapies for secondary hypertrophic pulmonary osteoarthropathy. Clin Rheumatol. 2011; 30:7–13.

2. Shim YW, Suh JS. Primary hypertrophic osteoarthropathy accompanied by Crohn's disease: a case report. Yonsei Med J. 1997; 38:319–322.

3. Yao Q, Altman RD, Brahn E. Periostitis and hypertrophic pulmonary osteoarthropathy: report of 2 cases and review of the literature. Semin Arthritis Rheum. 2009; 38:458–466.

4. Kim TH, Lee HH, Chung SH, et al. Complication of Crohn's disease on the vulva treated as a Bartholin's gland abscess: a case report. Korean J Obstet Gynecol. 2010; 53:657–660.

5. Neale G, Kelsall AR, Doyle FH. Crohn's disease and diffuse symmetrical periostitis. Gut. 1968; 9:383–387.

6. Kozak KR, Milne GL, Morrow JD, et al. Hypertrophic osteoarthropathy pathogenesis: a case highlighting the potential role for cyclo-oxygenase-2-derived prostaglandin E2. Nat Clin Pract Rheumatol. 2006; 2:452–456.

7. Johnson JC, Schmidt CR, Shrubsole MJ, et al. Urine PGE-M: a metabolite of prostaglandin E2 as a potential biomarker of advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2006; 4:1358–1365.

8. Dickinson CJ, Martin JF. Megakaryocytes and platelet clumps as the cause of finger clubbing. Lancet. 1987; 2:1434–1435.

9. Atkinson S, Fox SB. Vascular endothelial growth factor (VEGF)-A and platelet-derived growth factor (PDGF) play a central role in the pathogenesis of digital clubbing. J Pathol. 2004; 203:721–728.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download