Abstract
Background
To investigate the changes of cross sectional area (CSA) in paraspinal muscles upon magnetic resonance imaging (MRI) and bone mineral density (BMD) in postmenopausal osteoporotic spinal compression fractures.
Methods
We reviewed 81 postmenopausal women with osteoporosis, who had underwent MRI examination. The patients were divided into 51 patients who had osteoporotic spinal compression fractures (group I), and 30 patients who without fractures (group II). Group I were subdivided into IA and IB, based on whether they were younger (IA) of older (IB) than 70 years of age. We additionally measured body mass index and BMD. The CSA of multifidus, erector spinae, paraspinal muscles, psoas major (PT), and intervertebral (IV) discs were measured. The degree of fatty atrophy was estimated using three grades.
Results
The BMD and T-score of group I were significantly lower than those of group II. The CSA of erector spinae, paraspinal muscles, and PT in the group I was significantly smaller than that of group II. The CSA of paraspinal muscles in group IB were significantly smaller than those of group IA. The CSA of erector spinae, mutifidus, and PT in group IB were smaller than those of group IA, but the difference was not statistically significant. Group 1 exhibited greater fat infiltration in the paraspinal muscle than group II.
The current concept of sarcopenia was defined by Rosenberg (1989) as a condition involving a loss of muscle mass and strength due to age.[1] Age-related changes in body composition include decreased bone mineral density (BMD), sarcopenia, and hormonal fluctuations.[2] After 30 years of age, BMD decreases at an average rate of 1% per year. Muscle strength also declines during this time, with a gradual 10% loss from 25 to 50 years.[3] A progressive loss of muscle mass occurs from 40 years of age, which has been estimated at about 8% per decade until the age 70 years.[4] It is a complex medical condition that impacts on reduced mobility, increased disability, a higher risk of falls, reduced quality of life, increased health care costs, and ultimately, risk of mortality.[5-7]
Previous research has found the presence of an osteoporotic spinal fracture to be associated with reduced back extensor strength[8,9] decreased spinal mobility[10] and altered balance characteristics,[11,12] while few studies have addressed the changes of cross sectional area (CSA) in paraspinal muscles. Magnetic resonance imaging (MRI) and computerized tomography are the most specific techniques for the assessment of muscle mass or cross sectional muscle area.[13,14] Muscular strength is proportional to the CSA of the muscle, and measurement of the CSA of muscle is usually used to evaluate lumbar muscular strength.[15] Therefore, the aim of this study was to investigate the changes of CSA in paraspinal muscles upon MRI and BMD in postmenopausal osteoporotic spinal compression fractures.
We retrospectively reviewed 81 postmenopausal women with osteoporosis, who had underwent a conventional lumbar spine MRI examination from June 2009 to December 2012 in a single hospital. The patients were divided into 51 patients who had osteoporotic spinal compression fractures (group I), and 30 patients without osteoporotic spinal compression fractures (group II). Group I were subdivided into 21 patients who were under 70 years of age (IA), and 30 patients who were over 70 years of age (IB). We excluded patients who had vertebral fracture by high velocity injury, such as a motor vehicle accident or falling down from a height, and previous spine fusion operation. The BMD (mg/cm3) of all patients was measurement for the lumbar spine. In each group, we determined age, body mass index (BMI, kg/m2) based on weight and height, and compared the data with a control group (30 premenopausal women under 50 years of age with no spinal fractures. BMD of the thoracolumbar vertebrae was measured utilizing peripheral quantitative computed tomography (P-QCT; Somatom sensation 16, Simens, Erlangen, Germany), which enables the separate analyses of cortical, trabecular and total bone density, including T-score. Osteoporosis was defined as the BMD below 80 mg/cm3 and T-score below -2.5.
The axial scans were located through the lumbar intervertebral (IV) disc level using 1.5 Tesla MRI (Achieva; Philips Healthcare, Best, Netherlands). The images were pixel digital images with regions of interest (ROI) and they were stored in Picture Archiving and Communication System (PACS). The maximum anatomical CSA (mm2) was located between the L3/4 and L4/5 IV disc level in the neural posture, and the psoas major muscle (PT) was largest at the L4/5 IV disc level.[16] Therefore, the mean CSA from both sides of multifidus, erector spinae, paraspinal muscles (the multifidus and the erector spinae), PT, and disc were measured by drawing their outlines with ROI in the L4/5 IV disc level using axial T1 weighted MRI, which have been previously detailed.[16,17] The moment arm length (MAL, cm) was defined as the anterio-posterior distance between the center of the erector spinae and that of the IV disc (Fig. 1). Besides the measurement of the CSA, an assessment of fat composition can also be used to estimate the degree of atrophy of the paraspinal muscle.[18] The fat infiltration in the paraspinal muscle was measured semi-quantitatively with three visual scale grades at the L4/5 IV disc level.[19] The scale defined mild grade as a fat portion below 10%, moderate grade as 10-50% and severe grade as above 50% (Fig. 2). All measurements were independently performed by two orthopedics and one radiologist.
The result was obtained using independent t test and ANOVA. All values recorded in this study were presented as mean (standard deviation [SD]). SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical calculations. The Shapiro-Wilk test was performed in order to choose the appropriate statistical test for further analysis of the data. The data was normally distributed, and statistical significance was set at P<0.05 for all tests.
The mean age of group IA was 65.7 years (range, 60 to 69 years) and that of group IB was 77.7 years (range, 71 to 89 years). The mean age of group II was 67.4 years (range, 57 to 76 years) and that of the control group was 39.0 years (range, 20 to 50 years). The mean BMI of group IA was 22.2 kg/m2 (mean weight/mean height: 55.3 kg/157.9 cm), group IB was 22.1 kg/m2 (mean weight/mean height: 53.3 kg/155.2 cm), group II was 22.9 kg/m2 (mean weight/mean height: 56.8 kg/157.5 cm), and control group was 22.4 kg/m2 (mean weight/mean height: 58.7 kg/162.0 cm). The mean BMD and T-scores were: group IA, 56.6 mg/cm3 and -3.72; group IB, 50.9 mg/cm3 and -4.17; and group II, 70.4 mg/cm3 and -3.22.
The BMD values of group I were significantly lower than those of group II (P=0.000). Also, the T-score of group I was significantly lower than those of group II (P=0.000). The mean CSA of erector spinae, paraspinal muscles, and PT in the group IA and IB were significantly smaller than those of group II. However, the mean CSA of multifidus was not significantly different between the two groups. The CSA of IV disc and MAL were not significantly different between the two groups (Table 1).
The mean CSA of multifidus, paraspinal muscles, PT and CSA of IV disc in the group II were significantly smaller than that of control group. However, the mean CSA of erector spinae and MAL were not significantly different between the two groups (Table 2).
The BMD values of group IB were significantly lower than those of group IA (P=0.003). Also, the T-scores of group IB were significantly lower than those of group IA (P=0.001). The mean CSA of paraspinal muscles in group IB were significantly smaller than those of group IA (P=0.033). The mean CSA of erector spinae, multifidus, and PT in group IB were smaller than those of group IA, but the difference was not statistically significant. Rather, The CSA of IV discs was larger in group IB than in group IA with no statistically significantly different between the two groups (Table 3).
The fat infiltration grades in the paraspinal muscle of the control group were almost all mild grades, except for moderate grades determined in two patients. The numbers of patient of moderate and severe grades of group IA were 11 (52.4%) and 6 (28.6%) patients and those in group IB were 14 (46.7%) and 10 (33.3%) patients, and then severe grades of fat infiltration in the paraspinal muscle were more common in group I than in group II (Fig. 3).
In recent years, numerous data regarding the size and properties of muscle in patients with low back pain have been published.[19,20] Advancing age is associated with profound changes in the spine, such as atrophied lumbar paraspinal muscle with a reduction of CSA, biochemical degenerative change of IV disc with decrease disc height which dehydration and increased intramuscular fat infiltration.[21] In the present study, we measured the CSA of paraspinal muscle, psoas major, and IV disc and MAL of the erect spinae in the lumbar spine, in order to quantitatively evaluate muscle atrophy.
Previous research has found the presence of an osteoporotic spinal fracture to be associated with reduced back extensor strength,[8,9] decreased spinal mobility,[10] altered balance characteristics compared to individuals with osteoporosis, and no history of vertebral fracture.[11,12] However, there are only a few of published studies relating to the quantitative evaluation of back muscles in osteoporotic spinal compression fractures. Sinaki et al.[8] compared the extensor muscle strength of the spine of postmenopausal women with and without osteoporosis. They reported that strength was reduced in women with osteoporosis, and that this reduction increased with age. These data show that reductions in extensor muscle strength and postural abnormalities are associated with osteoporosis. Muscular imbalance may also favor the aggravation of the sagittal curvature of the vertebral spine and limit the range of motion.[9] Hyperkyphotic posture is a well-known complication of osteoporosis.[22] According to the lower back biomechanical model, the lumbar disc compression force and trunk muscle load depend on the MAL and CSA of the trunk muscles.[23] The elongation of MAL is effective in suppressing the compression force by reducing muscle activity. Vertebral fractures have been associated with increased segmental spinal loads and trunk muscle forces may be predicted through biomechanical models using optimization routines.[24] Women with osteoporosis had reduced flexibility and mobility that affected their walking and limited daily activities, contributing to a greater risk of falling and fracture.[11,12]
The CSA and fat content of the muscles have been evaluated by ultra-sonogram, computed tomography (CT), and MRI. MRI and CT are considered the golden standard and the most accurate imaging methods to assess muscle mass, muscle CSA and muscle quality, as determined by muscle density and intramuscular fat infiltration.[13,14] However, the high cost and operative complexity limit their use in clinical trials and clinical practice. In this study, MRI was carried out to confirm the diagnosis of osteoporotic spinal compression fractures.
Quantitative evaluation of back muscle strength involves measuring the CSA of the multifidus and the erector spinae, and measuring the isometric and isokinetic strength of the trunk muscles. Muscular strength is proportional to the CSA of the muscle (and is also associated with histologic characteristics, such as muscle composition and muscle fiber type), and measurement of the CSA of muscle is usually used to evaluate lumbar muscular strength.[15] Lee et al.[20] reported that the CSA of back muscle influenced the strength of trunk flexors and total trunk muscles in middle aged chronic low back pain patients. A limitation of this study was that muscle strength was not measured, but we did determine the CSA of muscle.
Sarcopenia is considered to be one of the main features of the aging process. It is characterized by a reduction in muscle mass and muscle strength, and affects women more than men.[25] Individuals with both osteoporosis and sarcopenia (the hazardous duet), so-called "sarco-osteoporosis", might be at higher fracture risk than those with osteoporosis or sarcopenia alone, especially if frail.[6,26] Associations with sarcopenia and osteopenia/osteoporosis in women have been reported in several studies.[27,28] Sarcopenia is associated with low BMD and osteoporosis in middle-aged and elderly men.[27] In Japan, sarcopenia is significantly associated with osteopenia and osteoporosis in a total 2,400 women.[28] As reports of osteoporotic fracture patients with sarcopenia are rare, we studied the association of osteoporotic spinal compression fractures with sarcopenia.
There were further limitations in this study. First, the small number of the subjects may have affected the statistical analysis. Second, this study is retrospective in nature, due to the sampling problems that are inherent to this mode of investigation.
In conclusion, postmenopausal osteoporotic spinal compression fracture results in profound changes in the spine, such as atrophied lumbar paraspinal muscle with a reduction of mean CSA, increased CSA of IV discs with aging, and increased intramuscular fat infiltration. This suggests that the women with osteoporotic spinal compression fractures had reduced paraspinal muscle volumes, resulting in a greater risk of falling and fracture.
Figures and Tables
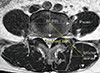 | Fig. 1Magnetic resonance axial image shows cross section of the muscles at L4/5 disc level. IV Disc: L4/5 intervertebral disc, moment arm length of the erector spinal muscle. |
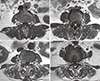 | Fig. 2MR axial images demonstrating fat infiltration in the lumbar paraspinal muscles. (A) normal (B) mild fatty atrophy of paraspinal muscles (C) moderate fatty atrophy of paraspinal muscles (D) severe fatty atrophy of paraspinal muscles. |
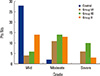 | Fig. 3The number of patients of each fat infiltration grade in the paraspinal muscle of each group. Pts, patients. |
References
1. American Society of Clinical Nutrition. Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1988. Am J Clin Nutr. 1989; 50:1121–1235.
2. Bayramoğlu M, Sözay S, Karataş M, et al. Relationships between muscle strength and bone mineral density of three body regions in sedentary postmenopausal women. Rheumatol Int. 2005; 25:513–517.

3. Riggs BL, Wahner HW, Melton LJ 3rd, et al. Rates of bone loss in the appendicular and axial skeletons of women. Evidence of substantial vertebral bone loss before menopause. J Clin Invest. 1986; 77:1487–1491.

5. Cruz-Jentoft AJ, Landi F, Topinková E, et al. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010; 13:1–7.

6. Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med. 2013; 49:111–117.
7. Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab. 2013; 20:1–10.

8. Sinaki M, Khosla S, Limburg PJ, et al. Muscle strength in osteoporotic versus normal women. Osteoporos Int. 1993; 3:8–12.

9. Cunha-Henriques S, Costa-Paiva L, Pinto-Neto AM, et al. Postmenopausal women with osteoporosis and musculoskeletal status: a comparative cross-sectional study. J Clin Med Res. 2011; 3:168–176.

10. Miyakoshi N, Hongo M, Maekawa S, et al. Factors related to spinal mobility in patients with postmenopausal osteoporosis. Osteoporos Int. 2005; 16:1871–1874.

11. Balzini L, Vannucchi L, Benvenuti F, et al. Clinical characteristics of flexed posture in elderly women. J Am Geriatr Soc. 2003; 51:1419–1426.

12. Keller TS, Harrison DE, Colloca CJ, et al. Prediction of osteoporotic spinal deformity. Spine (Phila Pa 1976). 2003; 28:455–462.

13. Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging. 2009; 13:724–728.

14. Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006; 83:1142–1148.

15. Maughan RJ. Relationship between muscle strength and muscle cross-sectional area. Implications for training. Sports Med. 1984; 1:263–269.
16. Jorgensen MJ, Marras WS, Gupta P. Cross-sectional area of the lumbar back muscles as a function of torso flexion. Clin Biomech (Bristol, Avon). 2003; 18:280–286.

17. Chaffin DB, Redfern MS, Erig M, et al. Lumbar muscle size and locations from CT scans of 96 women of age 40 to 63 years. Clin Biomech (Bristol, Avon). 1990; 5:9–16.

18. Ropponen A, Videman T, Battié MC. The reliability of paraspinal muscles composition measurements using routine spine MRI and their association with back function. Man Ther. 2008; 13:349–356.

19. Kang CH, Shin MJ, Kim SM, et al. MRI of paraspinal muscles in lumbar degenerative kyphosis patients and control patients with chronic low back pain. Clin Radiol. 2007; 62:479–486.

20. Lee HJ, Lim WH, Park JW, et al. The relationship between cross sectional area and strength of back muscles in patients with chronic low back pain. Ann Rehabil Med. 2012; 36:173–181.

21. Mengiardi B, Schmid MR, Boos N, et al. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: quantification with MR spectroscopy. Radiology. 2006; 240:786–792.

22. Sinaki M, Wollan PC, Scott RW, et al. Can strong back extensors prevent vertebral fractures in women with osteoporosis? Mayo Clin Proc. 1996; 71:951–956.

23. McGill SM. A myoelectrically based dynamic three-dimensional model to predict loads on lumbar spine tissues during lateral bending. J Biomech. 1992; 25:395–414.

24. Briggs AM, Wrigley TV, van Dieën JH, et al. The effect of osteoporotic vertebral fracture on predicted spinal loads in vivo. Eur Spine J. 2006; 15:1785–1795.

25. Crepaldi G, Romanato G, Tonin P, et al. Osteoporosis and body composition. J Endocrinol Invest. 2007; 30:42–47.
26. Buehring B, Krueger D, Binkley N. Effect of including historical height and radius BMD measurement on sarco-osteoporosis prevalence. J Cachexia Sarcopenia Muscle. 2013; 4:47–54.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download