Abstract
Background
Recently long-term safety of bisphosphonate raises issues about the duration of therapy. We examined the effects of a drug holiday (DH) on bone mineral density (BMD) and bone turnover markers.
Methods
In Korean, 125 women of 50 years of age or older with T-score≤-3.0 of their lumbar or left femoral BMD initiated bisphosphonate from 1999 based on retrospective chart review. 125 patients who had used bisphosphonate≥5 years started DH in 2006. Lumbar (L1-4), left femoral neck, total BMD, serum parameter (β-crossLaps [CTx], phosphorus, total calcium, total alkaline phosphatase), and urinary parameter (calcium/creatinine ratio) were measured before, the time of starting, and after DH.
Results
After DH, lumbar, femoral neck and total BMD did not change significantly (0.757±0.093→0.747±0.102, P=0.135, 0.567±0.079→0.560±0.082, P=0.351, 0.698±0.008→0.691±0.090 g/cm2, P=0.115, respectively). Serum CTx and total alkaline phosphatase were increased significantly (0.205±0.120→0.791±0.44 ng/mL, P<0.001, 54.52±13.40→60.42±15.543 IU/L, P=0.001, respectively). Urinary calcium/creatinine ratio increased significantly (0.132±0.076→0.156±0.093, P=0.012).
Conclusions
A DH could be cautiously considered in patients with long-term use of bisphosphonate if there is a concern about severe suppression of bone turnover with respect to long-term use because insignificant changes of BMD and significant increase of bone turnover markers are shown during the period.
Osteoporosis is a common metabolic bone disease with spectrum ranging from asymptomatic bone loss to disabling hip fracture. The objective of osteoporosis treatment is to maintain skeletal health and prevent bone fracture.[1] Bisphosphonate is the most commonly used medication to prevent and treat osteoporosis in Korea. Several studies have reported relationships between medication persistence and reduced fracture risk.[2-4] Highly persistent patients showed significant reduction of relative risk for all fractures, while poor compliers had higher and increased risk of fractures. Currently, bisphosphonate therapy raises issues about adverse events with many years of taking bisphosphonates - osteonecrosis of the jaw (ONJ), severely suppressed bone turnover, atrial fibrillation, subtrochanteric fracture, and esophageal cancer.[5-9] To avoid such adverse events of long-term treatment, stopping the medication after 5 years of usage and using anabolic agents & other schedule and doses of bisphosphonate were suggested.[10] Additionally drug holiday (DH) was also suggested since 1 year of DH was not associated with increase in vertebral and hip fractures[11] and even with one year after discontinuation of bisphosphonate treatment, spine and femoral neck bone mineral density (BMD) remained higher that placebo group.[12] The purpose of this study is to observe the effects of a 'DH' on BMD and bone turnover marker during bisphosphonate therapy in Korean women with osteoporosis.
The study was designed based on retrospective chart review and the study population consisted of 125 women aged 67.0 years old (50-81 years old) with osteoporosis (T-score≤-3.0 ; lumbar spine or femur of dual energy X-ray absorptiometry [DXA]) who initiated bisphosphonate between 1999 and 2004. The percentage of coefficient of variation (CV) of DXA (Hologic Discovery; HOLOGIC, Inc., Bedford, MA, USA) were 0.022% at L1-4, 0.046% at femoral neck, and 0.038% at total femur. If the patients had taken bisphosphonate more than 5 years, 1 year of "DH" was started in 2006. During the DH, bisphosphonate and calcium were at almost switched to alfacalcidol (0.5 µg) or calcitriol (0.25 µg), and calcium (158-600 mg) at almost patients. We prescribed one of three treatment regimens (daily, weekly, monthly) and divided these patients into alendronate (n=59), risedronate (n=58), and ibandronate group (n=8). And also lumbar and left femoral neck and total BMD, serum parameter (β-crossLaps [CTx; Roche Diagnostics, Mannheim, Germany, reference range 0.13-0.58 ng/mL], albumin, phosphorus, total calcium, and total alkaline phosphatase), and urinary parameters (fasting urine calcium, phosphorus, and creatinine) were measured 1 year before and after the DH. Non-parametric and paired T-test (Wilcoxon Signed Ranks Test) were used for comparing the parameters before and after the dug holiday using SPSS software version 20 (SPSS Inc., Chichago, IL, USA). Although the study was designed based on retrospective chart review, the study was started after the approval of Institutional Review Board (IRB).
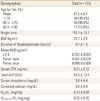
The baseline characteristics of the 125 patients are shown in Table 1. Mean age was 67.0±6.7 years, with mean BMI 23.1±2.9 kg/m2, and the duration of bisphosphonate therapy was average 6.1±1.3 years.
BMD was measured annually during bisphosphonate treatment. We compared the BMD at 1 year before starting DH, on the starting year, and 1 year after the DH for 125 patients who had taken bisphosphonate more than 5 years. Changes of lumbar (L1-4) BMD between the time of starting and 1 year after the DH was insignificant (0.757±0.093→0.747±0.102 g/cm2, P=0.135). Changes of femoral neck and total BMD were not significant (0.567±0.079 →0.560±0.082, P=0.351, 0.698±0.008→0.691±0.090 g/cm2, P=0.115 respectively (Fig. 1, Table 2).
After one year of DH, serum CTx and total alkaline phosphatase were increased significantly (0.21±0.12→0.79±0.44 ng/mL, P<0.001, 54.5±13.4→60.4±15.5 IU/L, P=0.001 respectively). Serum phosphorus was increased insignificantly (3.6±0.4→4.0±0.4 mg/dL, P=0.258). Corrected serum calcium was decreased significantly (9.0±0.4→8.9±0.8 mg/dL, P=0.02) (Table 2). Urinary calcium/creatinine ratio was increased significantly (0.132±0.08→0.16±0.09 mg/mg, P=0.012), but urinary phosphorus/creatinine ratio was insignificantly changed.
Osteoporosis is a common metabolic bone disease and an important cause of fracture in postmenopausal women. To achieve the maximum effects from bisphosphonate treatment, it is very important to keep taking medicine persistently. Compliance with bisphosphonate therapy is associated with a lower risk of osteoporotic fractures. Although long-term treatment with bisphosphonate has benefits on fracture reduction undoubtedly, there are some issues with respect to long-term safety. Since alendronate has received U.S. Food and Drug Administration (FDA) approval for the treatment of osteoporosis in 1995, oral alendronate (daily and weekly formulation in 1998, 2001 respectively) has begun to be used in Korea. Also risedronate, ibandronate and zoledronic acid have been approved and currently used thereafter.
Since ONJ,[5] a traumatic fractures due to severe suppressed bone turnover (SSBT),[6] atrial fibrillation,[7] subtrochanteric fracture,[8] and esophageal cancer,[9] were reported after the introduction of bisphosphonate for the treatment of osteoporosis, long-term safety of bisphosphonate use has been issued. With respect to long-term safety, previous reports were not problematic except 2 rare conditions, ONJ and atypical fractures.
Because bisphosphonate are tightly bound to bone after absorption and gradually released over months or years after treatment is stopped, which raises issues about both the possibility of stopping therapy and adverse events with long-term use, a DH was suggested concerning above possibilities.[13] A DH does not mean stopping the medication, but is a period between stop and restart of bisphophonate therapy. During DH, we switched from bisphosphonate and calcium to alfacalcidol (0.5 µg) or calcitriol (0.25 µg) and calcium (158-600 mg) because of approved drug for osteoporosis in Korea. Nevertheless, there is no strong evidence to provide guidance regarding how long to treat with bisphosphonate or how long the DH should continue.[14]
In this study, effects of 1 year of DH on BMD and bone turnover markers in postmenopausal osteoporosis using bisphosphonate for more than five years were observed. Our study shows spine and hip BMD changed insignificantly after the DH, but serum CTx and total alkaline phosphatase were increased significantly. Corrected calcium was decreased meaningfully, but level was within normal ranges. Urinary calcium/creatinine ratio was increased significantly. However, changes of bone turnover markers and BMD between 1 year before DH and the time of DH were observed not significantly. The results of insignificant changes of BMD and significant increase of serum CTx, total alkaline phosphatase, and urinary calcium/creatinine ratio after cessation of treatment suggest that the BMD is not influenced and increased bone remodeling recovers bone suppression after 1 year of DH. It is very hard to explain the decrease of urinary calcium/creatinine ratio at the time of starting DH compared with that of 1 year before DH, we could speculate that long-term use of bisphosphonate leading to bone suppression may cause reduction of calcium release from bone and decrease of urinary calcium excretion. In the subanalysis of alendronate, risedronate, and ibandronate group, there were no significant differences between three groups (date not shown). We Further studies will be needed to clarify unexplained result. Limitations of this study are; 1) there are no data comparing between the patients with DH and without DH, 2) bone resorption marker after the DH could not be compared with basal level before initiating bisphosphonate therapy, because urinary deoxypyridinoline/creatinine was only available as a bone markers when initiate treatment. However, change of bone resorption marker after the DH could be observed using serum CTx. In this study, 1 year off the drug is expected to affect no change of BMD, increase bone turnover markers, and be considered to have a positive effect on bone metabolism at least recovering the suppression of bone turnover due to long-term use of bisphosphonate, 3) since number of subjects in this study were small, more subjects will be needed to clarify the effects of DH.
Since at least one year of DH did not influence lumbar and femoral BMD, but significantly increased bone turnover markers, one year of DH could be considered cautiously concerning the severe suppression of bone turnover with respect to long-term safety of bisphosphonate use. This study would suggest the possibility of the DH after long period of bisphosphonate treatment.
Figures and Tables
Fig. 1
Changes of bone mineral density (BMD) in lumbar spine (1-4) and femur before and after the drug holiday (DH).

References
1. Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006. 119:S3–S11.

2. McCombs JS, Thiebaud P, McLaughlin-Miley C, et al. Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas. 2004. 48:271–287.

3. Caro JJ, Ishak KJ, Huybrechts KF, et al. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int. 2004. 15:1003–1008.

4. Siris ES, Harris ST, Rosen CJ, et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006. 81:1013–1022.

5. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003. 61:1115–1117.

6. Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005. 90:1294–1301.

7. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007. 356:1809–1822.

8. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007. 89:349–353.
9. Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009. 360:89–90.

11. Sebba A. Osteoporosis: how long should we treat? Curr Opin Endocrinol Diabetes Obes. 2008. 15:502–507.

12. Watts NB, Chines A, Olszynski WP, et al. Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int. 2008. 19:365–372.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download