Abstract
Objectives
Although the coating of surface sealants to dental composite resin may potentially reduce bacterial adhesion, there seems to be little information regarding this issue. This preliminary in vitro study investigated the adhesion of Streptococcus mutans (S. mutans) on the dental composite resins coated with three commercial surface sealants.
Materials and Methods
Composite resin (Filtek Z250) discs (8 mm in diameter, 1 mm in thickness) were fabricated in a mold covered with a Mylar strip (control). In group PoGo, the surfaces were polished with PoGo. In groups PS, OG, and FP, the surfaces polished with PoGo were coated with the corresponding surface sealants (PermaSeal, PS; OptiGuard, OG; Fortify Plus, FP). The surfaces of the materials and S. mutans cells were characterized by various methods. S. mutans adhesion to the surfaces was quantitatively evaluated using flow cytometry (n = 9).
Results
Group OG achieved the lowest water contact angle among all groups tested (p < 0.001). The cell surface of S. mutans tested showed hydrophobic characteristics. Group PoGo exhibited the greatest bacterial adhesion among all groups tested (p < 0.001). The sealant-coated groups showed statistically similar (groups PS and FP, p > 0.05) or significantly lower (group OG, p < 0.001) bacterial adhesion when compared with the control group.
Proper finishing and polishing of dental restorations is desirable for aesthetic considerations and maintenance of oral health. A rough composite resin surface may decrease the gloss and esthetic appearance as well as increase the number of sites on the restoration surface prone to bacterial biofilm accumulation, which leads to increased risk of both caries and periodontal inflammation.12 In dental biofilm formation, Streptococcus mutans (S. mutans) is known to be primarily responsible for the initiation of tooth decay as well as for the progression of an established lesion.3 The initial adhesion of specific bacteria to tooth or dental restoration surfaces is the essential prerequisite for the formation of a cariopathogenic biofilm within the oral cavity.3
Restorative covering agents, or ‘surface sealants’, are low-viscosity resins polymerized onto composite resin surfaces after penetrating and filling, through capillary action, into the microstructural defects left from the finishing and polishing processes.24 It has been reported that the materials maintain surface smoothness,5 improve wear resistance,46 and ensure good marginal sealing7 of the composite restoration. Thus, the application of specific surface sealants may be a useful clinical procedure to improve the surface quality of composite resin restorations.8 However, the use of sealants to maintain the smoothness of resin surfaces is still questionable.2
In general, a finished/polished or surface sealant-coated composite resin should have low susceptibility to adhere to oral microorganisms.3 The physicochemical surface characteristics, such as surface roughness, hydrophobicity, and surface free energy (SFE), of dental restorations significantly affect the adhesion of oral bacteria to the surfaces.9 However, there seems to be little information regarding bacterial adhesion tendency onto dental composite resin coated with surface sealants.
The purpose of this in vitro study was to investigate the adhesion of S. mutans on the dental composite resins coated with three commercial surface sealants using flow cytometry (FCM), and to correlate these findings to the surface characteristics of the coating materials. Results were compared to those for surfaces finished with Mylar strip and polished with a commercial one step polishing disc (PoGo, Dentsply/Caulk, Milford, DE, USA). The null hypothesis tested was that the surface sealant applications would not reduce initial adhesion of S. mutans to composite resin.
Microhybrid composite resin (Filtek Z250, 3M ESPE, St. Paul, MN, USA, A2 shade) was used as the substrate. Two unfilled (PS, PermaSeal, Ultradent Products Inc., South Jordan, UT, USA; OG, OptiGuard, Kerr Corp., Orange, CA, USA) and one microfilled (FP, Fortify Plus, Bisco Inc., Schaumburg, IL, USA) sealants were investigated. Their codes, manufactures, lot numbers, main compositions, and application methods are summarized in Table 1.
Cylindrical molds (8 mm in diameter, 1 mm in height) were placed on a Mylar strip (KerrHawe SA, Bioggio, Switzerland) over a glass slide. The composite resin Filtek Z250 was filled into the mold, covered with another polyester strip and glass slide, gently pressed to expel the excess material, and light-irradiated for 20 seconds by placing the tip of the light guide of a light-curing unit (Elipar TriLight, 3M ESPE, St. Paul, MN, USA, standard mode, output intensity = 750 mW/cm2) against the upper glass. The irradiation procedure was performed on the other side of the specimen. The cured specimens were then stored in a dry condition at a temperature of 37℃.
After 24 hours, the composite resin disc specimens were removed from the mold and randomly divided into six groups depending on the post-treatment method. In group Mylar, the surfaces were finished as they were with a Mylar film. In group PoGo, the surfaces were ground wet with 1,200 grit wet silicon carbide paper and then polished with the flat, broad surface of the PoGo diamond micro-polisher disc according to the manufacturer's instructions,10 followed by careful rinsing/drying procedures. In groups PS, OG, and FP, the surfaces were also ground and polished as described above, then followed by careful rinsing/drying procedures. A thin coat of the corresponding surface sealants was then applied to the surfaces and gently air-thinned. The surface sealant-coated surfaces were covered with polyester strip and glass slide, and light-cured by placing the light guide tip of the curing light (Elipar TriLight) against the upper glass. The application of the surface sealants was performed according to the respective manufacturers' instructions (Table 1). After storage in a dry condition at 37℃ for 24 hours, the polyester strips were removed from the specimen. The specimen preparation procedures were performed on a clean bench.
One representative specimen of each group was prepared for the scanning electron microscope (SEM). Specimens were mounted in aluminum stubs, sputter-coated with platinum, and examined using a SEM (JSM-6700F, JEOL, Tokyo, Japan). The average surface roughness Ra of each specimen was determined using a previously calibrated profilometer (Surftest SV-400, Mitutoyo Corp., Kawasaki, Japan) at a stylus speed of 0.1 mm/sec, a cutoff of 0.8 mm, and a range of 600 µm. The Ra value of each specimen was recorded as the average of the five readings (n = 6/group). The contact angle (CA) of water droplets on the specimen surfaces was determined by the sessile drop method using a CA goniometer (OCA 15 plus, DataPhysics Instrument GmbH, Filderstadt, Germany) (n = 6/group).
Freeze-dried strains of S. mutans (ATCC 25175, KCTC 3065) were recovered and seeded on brain heart infusion (BHI) agar plates and cultivated for 48 hours in a micro-aerobic environment (37℃) created by an anaerobic cultivation system (Anoxomat Mark II, MART Microbiology B.V., Lichtenvoorde, The Netherlands). An isolated single colony was then inoculated to fresh BHI and incubated for 12 hours to adjust the optical density (OD) of the bacterial cell suspension to 0.3 at 600 nm, then checked using a ultraviolet-visible spectrophotometer (UV-1650PC, Shimadzu, Kyoto, Japan).
Cell-surface hydrophobicity of S. mutans was assessed by the microbial adhesion to hydrocarbon test.1112 The prepared bacterial cell suspension was washed twice and suspended in sterile saline (0.85%) so that its optical density was 0.3 at 600 nm. Thereafter, 3.0 mL of the bacterial cell suspension was placed in glass test tubes, and varying volumes of either hexadecane or toluene test hydrocarbon (0, 0.025, 0.05, 0.1, 0.2, 0.3, and 0.4 mL) were added. The glass tubes were agitated uniformly in a vortex mixer for 2 minutes and allowed to equilibrate at room temperature for 10 minutes. After the hydrocarbon phase had been separated from the aqueous phase, the OD of the aqueous phase was determined at 600 nm. The hydrophobicity index, expressed as a percentage, was calculated as: [(ODinitial - OD final) / ODinitial] × 100. S. mutans with a hydrophobicity index greater than 70% was classified as hydrophobic.11
The composite resin specimens were placed into a 24-well plate with one specimen per well, and 2 mL of the bacterial suspension was added into each well. The well plates were incubated at 37℃ for 2.5 hours to allow the S. mutans cells to attach to the specimen surfaces.13 The incubation time was chosen because initial biofilm formation in the oral cavity normally occurs in 2 - 4 hours.14 After incubation, the test specimens were washed twice with phosphate-buffered saline (PBS) to remove the non-adhering cells. Each specimen was then transferred to a microtube containing 1 mL of PBS. The tubes were ultrasonicated using four 30 seconds pulses with three 30 seconds intermittent coolings to detach bacteria adhered to the resin specimen surfaces.15 The detached cells were washed four times with 0.85% saline solution, then finally stained using the LIVE/DEAD BacLight Bacterial Viability and Counting Kit (L34856, Molecular Probes, Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions.16 Briefly, 10 µL of the bacterial cell suspension was mixed with 986 µL of 0.85% saline solution in an FCM tube. Then, 1 µL of reference microsphere beads, 1.5 µL of 3.34 mM SYTO 9, and 1.5 µL of 20 mM PI were added into the tube. The tube was incubated at room temperature in the dark for 15 minutes.16
FCM was performed using an Accuri C6 flow cytometers (Accuri Cytometers, Inc., Ann Arbor, MI, USA) with a 488 nm excitation from a blue solid-state laser at 50 mW.17 Fluorescence filters and detectors were all standardized with green fluorescence collected in the FL1 channel (530 ± 15 nm) and red fluorescence collected in the FL3 channel (> 670 nm).17 All parameters were collected as logarithmic signals. The flow rate of the samples was adjusted to keep the event rate below 5,000 events per second. At least 10,000 cells for each sample were counted. Data were analyzed using CFlow Plus software (Accuri Cytometers, Inc.). In FCM dot plots, the distinct bacterial populations (L, live cells; D, dead cells; B, microsphere bead) were gated on the basis of the different viability stages.1617 The bacterial cells were enumerated by comparison to a known number of counting beads.16 Total (red and green) counts of bacteria per unit area (mm) were calculated for each specimen.18 These experiments were run in triplicate and repeated three separate times (n = 9).
In addition, fluorescence microscopic visualization of the adherent S. mutans on specimens was performed. The bacteria-adhered specimens were stained using the same LIVE/DEAD staining kit and incubated for 15 minutes at room temperature in the dark. The dye solution was removed, and the images of the cells were observed with a fluorescence microscope (BX53, Olympus, Center Valley, PA, USA).
The statistical analyses were carried out using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA) at a significance level of 0.05. All data were examined for the normality of distribution and the equality of variances with the Shapiro-Wilk test and the Levene test, respectively. The adhesion data were log10 transformed to meet homogeneity of variance before analysis. Statistical analysis of the data was performed using a one-way analysis of variance followed by Tukey's post hoc test. In addition, a post hoc power analysis was performed to examine the power of the adhesion data using G*Power 3 software. A power of 0.80 was regarded as acceptable.
Figure 1 shows the representative scanning electron microscopy (SEM) surface images for each group. The surface roughness and water contact angle (CA) values are summarized in Table 2. A smooth surface was obtained against the Mylar strip. The surface polished with the PoGo system showed a significantly higher average surface roughness (Ra) value than the control Mylar group (p < 0.001). The surfaces coated with the surface sealants exhibited smoothness again, their Ra values decreasing to a level statistically similar to that of the control (p > 0.05). Group PoGo showed a significantly lower CA than the control Mylar group (p = 0.017). Group OG achieved the lowest CA value among all groups tested, the difference being statistically significant (p < 0.001).
Figure 2 shows the adherence of S. mutans to the hydrocarbons as a function of increasing hydrocarbon volume. Each hydrocarbon was added to an aqueous suspension of S. mutans, mixed for 2 minutes, and allowed to stand. Adherent cells rose with the hydrocarbon, forming a creamy upper layer and a clear aqueous phase. S. mutans showed high affinity for the two test hydrocarbons, over 80% of the cells being removed from the aqueous phase by 0.3 mL hexadecane or toluene. This indicates the hydrophobic characteristics of the cell surface of S. mutans tested in this study.
Figure 3 shows quantification of S. mutans adhesion on the resin specimens using FCM. Based on a post hoc power analysis, the power values for the data were higher than 0.80 (π = 1.00). Group PoGo achieved the greatest bacterial adhesion among all groups tested, the difference being statistically significant (p < 0.001). Groups PS and FP showed statistically similar adhesions to the control group (p > 0.05). Group OG showed significantly lower bacterial adhesion than the other two sealant-treated groups (p < 0.05). Representative fluorescence microscope images of S. mutans adhered on the resin specimens are shown in Figure 4, a similar tendency being found with FCM.
Ideally, final composite resin restoration surfaces prepared by a clinician should have a low susceptibility to adhere to oral bacteria. Bacterial adhesion and retention to a dental substrate surface take places in four phases: transport of the bacterium toward the surface, initial adhesion, attachment by specific interactions, and colonization to form a biofilm.119 In initial bacterial adhesion and retention, a bacterium and the surface physicochemical properties interact with each other from a certain distance through a combination of van der Waal's attractive forces and electrostatic repulsive forces.1 Bacteria on rough surfaces are more protected against shear forces and can, thereby, have the necessary time to reach direct contact or to bridge the distance.1 Thus, in vitro bacterial adhesion is primarily influenced by surface characteristics of materials, particularly surface roughness and SFE (Table 2). The findings of this in vitro study (Figures 3 and 4) suggest that the application of surface sealants reduces initial S. mutans adhesion to composite resin polished with the PoGo one step polishing system. Therefore, the null hypothesis that surface sealant applications would not reduce initial adhesion of S. mutans to composite resin was partially rejected.
In this in vitro study, FCM was used to quantify the bacterial adhesion to resin specimens (Figure 3). The colony-forming unit-counting method, though a conventional method for bacterial cell counting, is labor-intensive and time-consuming mainly due to the slow growing nature of the organism. FCM can overcome these obstacles, thereby allowing more rapid and easier assessment of the viability of bacteria.20 Moreover, plate count numbers represent only culturable cells, whereas the FCM technique can detect and discriminate live culturable, live nonculturable, and dead cells.21 For FCM, in this study, LIVE/DEAD cell staining was performed to differentiate viable and nonviable bacterial cells, according to cytoplasmic membrane permeability.1822 This staining method uses two separate dyes: SYTO 9, which has a green fluorescence emission and stains both live and dead bacterial DNA, and propidium iodide (PI), which has a red fluorescence emission and penetrates only damaged cell membranes.23
Similar to the Z250 composite resin, the surface sealants primarily consist of hydrophobic resin monomers such as bisphenol-A glycidyl dimethacrylate (Bis-GMA), triethylene glycol dimethacrylate (TEGDMA), and urethane dimethacrylate (UDMA) (Table 1). Although the monomers are hydrophobic, they have hydrophilic functional groups (e.g., hydroxyl, ethylene oxide, and urethane groups, respectively) that can serve as sites for water absorption.24 Therefore, the surface of the surface sealants may have different chemical reactivity or hydrophobicity depending on their resin composition. The water CA values indicate that OG had a significantly hydrophilic surface compared to PS and FP, perhaps due to the higher hydrophilicity of TEGDMA, the resin monomer of OG, as compared with Bis-GMA or UDMA.2526 In this study, the hydrophobicity of the cell surface of the S. mutans strain used was also investigated. The affinity of S. mutans towards the test hydrocarbons as a function of hydrocarbon volume (Figure 2) clearly shows that the cell surface has hydrophobic characteristics (hydrophobicity index approximately 80%).
Polished restorations should demonstrate an enamel-like surface texture and gloss.10 The smoothest obtainable surface on composite resin restorations is achieved by curing the material in direct contact with a smooth matrix surface (Mylar strip).1027 In this study, the Mylar-finished composite resin (control) showed smooth surface with a mean Ra of 0.05 µm (Table 2). In a previous study,10 PoGo produced comparable surface roughness for two composite resins tested (microhybrid type Esthet-X, Dentsply/Caulk; microfilled type Clearfil ST, Kuraray Europe GmbH, Dusseldort, Germany), with only one disc and a short application time of 30 seconds. On the contrary, in other studies,2829 PoGo polishing exhibited a significantly higher Ra value than Mylar finishing for the Z250 microhybrid composite resin. This indicates that the surface roughness of a polished composite resin depends on the size, hardness, and amount of filler particles.28 In this study, group PoGo showed a significantly higher Ra value than group Mylar. Changes in surface roughness can also influence the CA, thereby changing surface energy characteristics.30 The PoGo-polished surface showed a significantly lower CA value than the control Mylar-finished surface, indicating more hydrophilic surface.31
Previous in vivo studies suggested a threshold surface roughness for bacterial retention (Ra = 0.2 µm).32 An increase in surface roughness above this threshold may result in greater plaque accumulation, thereby increasing the risk for both caries and periodontal inflammation.8 In this study, the PoGo-polished surface significantly increased S. mutans adhesion when compared with the Mylar-finished surface (Figures 3 and 4), probably due to the increased surface roughness (slightly over 0.2 µm in Ra).11933
Following the application of the surface sealants, the Ra value of the PoGo-polished surface significantly decreased to a level statistically similar to that of the control Mylar group. The representative SEM images (Figure 1) clearly show that a surface treated with sealants contains substantially fewer voids, cracks, and other types of microstructural defects compared with the PoGo-polished surface.8 Surface sealants may wet the internal surfaces of microstructural defects due to their low viscosity and high wettability.34 There were no significant differences in surface roughness among the three surface sealant-coated groups (Table 2). In a previous study,8 a filled surface sealant showed a significantly higher Ra value than an unfilled one, which is not in accordance with the results of this study, perhaps because the surface sealant-coated composite resin surfaces were prepared against Mylar strips in this study. Except for group PoGo, there were only small variations in the Ra value. According to Busscher et al.,35 changes in solid surface Ra below 0.1 µm have little effect on CA. Thus, except for group PoGo, it can be assumed that surface roughness did not affect CAs and that the CA data reflect the inherent chemical reactivity of the surfaces.3036
Treatment of PoGo polished composite resins with surface sealants significantly decreased the adhesion of S. mutans (Figures 3 and 4), probably due to its enhanced surface smoothness (Ra < 0.2 µm, Table 2).32 Although there were no significant differences in Ra value among the three surface sealant groups, the more hydrophilic surface (group OG) showed a significantly decreased adhesion of S. mutans that showed hydrophobic characteristics (Figure 2) than the more hydrophobic surfaces (groups PS and FP). This finding is in sharp contrast with that by Buergers et al.,37 in which a silorane-based composite showed low streptococcal adhesion potential as compared with methacrylate composite resins due to its increased hydrophobicity. However, the correlation of CA (or surface hydrophobicity) and bacterial adhesion has also been critically discussed.3738 Surfaces with increased hydrophobicity are assumed to be more resistant against attack by water or water-soluble species.38 The cell surface of the tested S. mutans strain was hydrophobic and, as a result, had a low SFE. Thus, it was hypothesized that primary adhesive forces may rise for increased hydrophobic surfaces because water is more easily removed from the area between the S. mutans cell surface and a hydrophobic material than from the area between the cell surface and a hydrophilic material, enabling a closer approach and thus stronger adhesion forces.3839 Although the PoGo-polished surface was more hydrophilic than the Mylar-finished surface, the former showed a significantly increased S. mutans adhesion than the latter. Surface roughness and SFE interact with each other, but the influence of surface roughness overrules that of the SFE when the surface is sufficiently rough (Ra > 0.2 µm).19
The findings of this in vitro study suggest that the application of surface sealants aids in reducing S. mutans adhesion potential to composite resin polished with the PoGo one step polishing system. Moreover, the OG-coated surface showed a significantly decreased S. mutans adhesion than the Mylar-finished surface (Figure 3). These findings confirm that rough surfaces (Ra > 0.2 µm) demonstrate the predominance of roughness (group PoGo), and that smooth surfaces (Ra < 0.2 µm) represent the influence of the SFE (group OG).19 However, caution should be used when generalizing the results directly to the clinical situation. The present in vitro study tested only the initial physicochemical interaction phase of bacterial adhesion. Moreover, only one S. mutans strain was tested, although the oral cavity is constantly contaminated by many diverse microbial species.1 The influence of acquired pellicle, which can mask the physicochemical surface properties of materials,33 was not included in this study. In addition, the surface sealant-coated composite resin surfaces were prepared against Mylar strips, instead of using a brush for coating as in dental practice, to compare the S. mutans adhesion performance of the sealants based on their different chemical compositions (Table 1).
Recent composite resins such as nanofilled composite may provide smoother surface after polishing without surface sealants.2 Moreover, the surface sealant placed on smooth, polished areas will eventually wear away due to prolonged exposure to thermal and abrasive stimuli, which may necessitate reapplication of sealant.7 Further in vitro and clinical studies are needed to determine the short- and long-term merit of placement of surface sealants on polished composite resin.
Within the limitations of this in vitro study, the application of the surface sealants effectively reduced S. mutans adhesion to the composite resin polished with the PoGo one-step polishing system. The sealant-coated groups showed statistically similar or significantly lower bacterial adhesions when compared with the Mylar-finishing (control) group.
Figures and Tables
 | Figure 1Representative scanning electron microscopy images of the composite resin surfaces finished with Mylar (a), polished with PoGo (b), and coated with PermaSeal (c), OptiGuard (d), and Fortify Plus (e), respectively (original magnification ×5,000, bar = 2 µm). |
 | Figure 2Affinity of S. mutans towards the test hydrocarbons (closed circles, hexadecane; open triangles, toluene) as a function of hydrocarbon volume. Aqueous bacterial suspensions were mixed with varying volumes of hydrocarbon. Results are expressed as a percentage of the initial absorbance of the aqueous suspension as a function of hydrocarbon volume. The photo inside the graph shows adherence of S. mutans to hexadecane as a function of increasing hexadecane volume. From left to right: 0, 0.025, 0.05, 0.1, 0.2, 0.3, and 0.4 mL hexadecane added. When toluene was substituted for hexadecane, a similar trend was observed. S. mutans, Streptococcus mutans. |
 | Figure 3Quantification of S. mutans adhesion for each group using flow cytometry. (a) Flow cytometry dot plots; (b) Bacterial counts per unit area for each group (n = 9) (green, live bacteria; red, dead bacteria). The vertical bar indicates standard deviation. Mean values with different superscripts (a, b, and c) are significantly different (p < 0.05). Means were log10 transformed prior to analysis. L, live bacteria; D, dead bacteria; B, reference microsphere beads; S. mutans, Streptococcus mutans. |
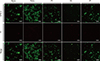 | Figure 4Representative fluorescence microscope images of S. mutans adhered on the resin specimens. SYTO 9-labeled live bacteria images (upper), propydium iodide-labeled dead bacteria images (central), and merged images of LIVE/DEAD bacteria (lower) (original magnification ×100, bar = 200 µm). S. mutans, Streptococcus mutans; PI, propydium iodide; PS, PermaSeal (Ultradent Products Inc.); OG, OptiGuard (Kerr Corp.); FP, Fortify Plus (Bisco Inc.). |
Table 1
Composition and application protocol of the composite resin and three surface sealants used

Table 2
Means and standard deviations of surface roughness and contact angle for each group (n = 6)

Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2061732).
References
1. Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 1997; 13:258–269.

2. Cilli R, de Mattos MC, Honorio HM, Rios D, de Araujo PA, Prakki A. The role of surface sealants in the roughness of composites after a simulated toothbrushing test. J Dent. 2009; 37:970–977.

3. Bürgers R, Cariaga T, Müller R, Rosentritt M, Reischl U, Handel G, Hahnel S. Effects of aging on surface properties and adhesion of Streptococcus mutans on various fissure sealants. Clin Oral Investig. 2009; 13:419–426.

4. Dickinson GL, Leinfelder KF, Mazer RB, Russell CM. Effect of surface penetrating sealant on wear rate of posterior composite resins. J Am Dent Assoc. 1990; 121:251–255.

5. dos Santos PH, Consani S, Correr Sobrinho L, Coelho Sinhoreti MA. Effect of surface penetrating sealant on roughness of posterior composite resins. Am J Dent. 2003; 16:197–201.
6. Prakki A, Ribeiro IW, Cilli R, Mondelli RF. Assessing the tooth-restoration interface wear resistance of two cementation techniques: effect of a surface sealant. Oper Dent. 2005; 30:739–746.
7. Ramos RP, Chimello DT, Chinelatti MA, Dibb RG, Mondelli J. Effect of three surface sealants on marginal sealing of Class V composite resin restorations. Oper Dent. 2000; 25:448–453.
8. dos Santos PH, Pavan S, Suzuki TY, Briso AL, Assunção WG, Sinhoreti MA, Correr-Sobrinho L, Consani S. Effect of fluid resins on the surface roughness and topography of resin composite restorations analyzed by atomic force microscope. J Mech Behav Biomed Mater. 2011; 4:433–439.

9. An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998; 43:338–348.

10. Türkün LS, Türkün M. The effect of one-step polishing system on the surface roughness of three esthetic resin composite materials. Oper Dent. 2004; 29:203–211.
11. Martin MA, Pfaller MA, Massanari RM, Wenzel RP. Use of cellular hydrophobicity, slime production, and species identification markers for the clinical significance of coagulase-negative staphylococcal isolates. Am J Infect Control. 1989; 17:130–135.

12. Nostro A, Cannatelli MA, Crisafi G, Musolino AD, Procopio F, Alonzo V. Modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett Appl Microbiol. 2004; 38:423–427.

13. Zhou L, Tong Z, Wu G, Feng Z, Bai S, Dong Y, Ni L, Zhao Y. Parylene coating hinders Candida albicans adhesion to silicone elastomers and denture bases resin. Arch Oral Biol. 2010; 55:401–409.

14. Montanaro L, Campoccia D, Rizzi S, Donati ME, Breschi L, Prati C, Arciola CR. Evaluation of bacterial adhesion of Streptococcus mutans on dental restorative materials. Biomaterials. 2004; 25:4457–4463.

15. Kang SH, Lee HJ, Hong SH, Kim KH, Kwon TY. Influence of surface characteristics on the adhesion of Candida albicans to various denture lining materials. Acta Odontol Scand. 2013; 71:241–248.

16. Orth R, O'Brien-Simpson N, Dashper S, Walsh K, Reynolds E. An efficient method for enumerating oral spirochetes using flow cytometry. J Microbiol Methods. 2010; 80:123–128.

17. Pan H, Feng J, Cerniglia CE, Chen H. Effects of Orange II and Sudan III azo dyes and their metabolites on Staphylococcus aureus. J Ind Microbiol Biotechnol. 2011; 38:1729–1738.

18. Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009; 37:289–296.

19. Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995; 22:1–14.

20. Burdz TV, Wolfe J, Kabani A. Evaluation of sputum decontamination methods for Mycobacterium tuberculosis using viable colony counts and flow cytometry. Diagn Microbiol Infect Dis. 2003; 47:503–509.

21. Gunasekera TS, Attfield PV, Veal DA. A flow cytometry method for rapid detection and enumeration of total bacteria in milk. Appl Environ Microbiol. 2000; 66:1228–1232.

22. Filoche SK, Coleman MJ, Angker L, Sissons CH. A fluorescence assay to determine the viable biomass of microcosm dental plaque biofilms. J Microbiol Methods. 2007; 69:489–496.

23. Cai Y, Strømme M, Welch K. Bacteria viability assessment after photocatalytic treatment. 3 Biotech. 2014; 4:149–157.

24. Antonucci JM, Fowler BO, Dickens SH, Richards ND. Novel dental resins from trialkoxysilanes and dental monomers by in situ formation of oligomeric silyl ethers and silsesquioxanes. Polym Prepr. 2002; 43:633–634.
25. Pereira SG, Osorio R, Toledano M, Nunes TG. Evaluation of two Bis-GMA analogues as potential monomer diluents to improve the mechanical properties of light-cured composite resins. Dent Mater. 2005; 21:823–830.

26. Tay FR, Pashley DH, Kapur RR, Carrilho MR, Hur YB, Garrett LV, Tay KC. Bonding BisGMA to dentin-a proof of concept for hydrophobic dentin bonding. J Dent Res. 2007; 86:1034–1039.

27. Wilson F, Heath JR, Watts DC. Finishing composite restorative materials. J Oral Rehabil. 1990; 17:79–87.

28. Ozel E, Korkmaz Y, Attar N, Karabulut E. Effect of one-step polishing systems on surface roughness of different flowable restorative materials. Dent Mater J. 2008; 27:755–764.

29. Korkmaz Y, Ozel E, Attar N, Aksoy G. The influence of one-step polishing systems on the surface roughness and microhardness of nanocomposites. Oper Dent. 2008; 33:44–50.

30. Kim MJ, Kim YK, Kim KH, Kwon TY. Shear bond strengths of various luting cements to zirconia ceramic: surface chemical aspects. J Dent. 2011; 39:795–803.

31. Hay KM, Dragila MI, Liburdy J. Theoretical model for the wetting of a rough surface. J Colloid Interface Sci. 2008; 325:472–477.

32. Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996; 7:201–211.

33. Claro-Pereira D, Sampaio-Maia B, Ferreira C, Rodrigues A, Melo LF, Vasconcelos MR. In situ evaluation of a new silorane-based composite resin's bioadhesion properties. Dent Mater. 2011; 27:1238–1245.

34. Kawai K, Leinfelder KF. Effect of surface-penetrating sealant on composite wear. Dent Mater. 1993; 9:108–113.

35. Busscher HJ, van Pelt AWJ, de Boer P, de Jong HP, Arends J. The effect of surface roughening of polymers on measured contact angles of liquids. Colloids Surf. 1984; 9:319–331.

36. Kim YK, Son JS, Kim KH, Kwon TY. Influence of surface energy parameters of dental self-adhesive resin cements on bond strength to dentin. J Adhes Sci Technol. 2013; 27:1778–1789.

37. Buergers R, Schneider-Brachert W, Hahnel S, Rosentritt M, Handel G. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent Mater. 2009; 25:269–275.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download