Abstract
Paraformaldehyde has been used in the past as a pulpotomy agent. However, it has a severe cytotoxic effect and may cause alveolar bone necrosis. Depulpin, a devitalizing agent containing 49% paraformaldehyde, is no longer used frequently due to its severe side effects. In the two cases described in the present study, Depulpin was used as a devitalizing agent during root canal treatment. It caused a gradual loss of sensibility in adjacent teeth, gingival necrosis, and osteomyelitis. This case report demonstrates the serious side effects of using a paraformaldehyde-containing paste as a devitalizing agent for pulp, particularly mandibular bone necrosis.
Successful local anesthesia and performing pain-free root canal treatment may be a challenge for dentists, as it is extremely difficult to achieve profound anesthesia during endodontic treatment. In particular, block anesthesia for endodontic treatment on the mandibular molar is associated with a high risk of failure. A study by Cohen et al. reported that 39% of patients who had irreversible pulpitis of the mandibular molar remained sensitive to a cold test after administration of an inferior alveolar nerve block with 2% lidocaine HCl.1
Depulpin (Voco GmbH, Cuxhaven, Germany) was introduced as a medicament to address this issue. Depulpin contains 49% paraformaldehyde, which causes devitalization of pulp. However, the safety of paraformaldehyde has not been verified. Some case reports have described the cytotoxic effects of paraformaldehyde-containing paste.234 The side effects of Depulpin may be reduced by following the manufacturer's instructions, which recommend application only on the vital pulp, use of a small amount (20 - 25 mg/tooth), and use on a class I cavity that can attain a complete seal. In 2013, the Ministry of Food and Drug Safety of Korea issued a recommendation to use Depulpin with caution because of safety issues. Storage or usage of expired Depulpin has been prohibited in Korea since February 2014.
The purpose of this article is to present the severe side effects of paraformaldehyde-containing paste on periodontal tissues and to recommend a restricted use of it.
A 57 year old man visited the Department of Conservative Dentistry with discomfort around the left lower posterior tooth (tooth #36) that was undergoing root canal treatment. He stated that nonsurgical endodontic treatment was initiated on tooth #36 in a private clinic because of severe spontaneous pain 2 weeks before. He had received a temporary crown on tooth #36 and did not exhibit any symptoms at the time. He felt spontaneous pain on tooth #36 after crown fracture due to mastication of hard food. His medical history was non-contributory. When the patient visited our clinic, he complained of spontaneous throbbing pain in tooth #36. A clinical examination revealed sensitivity to percussion on teeth #34, 35, and 36. Periodontal probing of the mesial gingiva on tooth #36 showed a 5 mm pocket and appearance of slight alveolar bone loss. Increased periodontal space on tooth #36 was observed, and periapical radiography indicated perforation of the pulpal floor of the tooth (Figures 1a and 1d). When the temporary filling material was removed, perforation of the pulpal floor, in which Depulpin had been placed, was confirmed (Figures 1b and 1c). Previously initiated therapy and symptomatic apical periodontitis were diagnosed for tooth #36. After canal negotiation, the perforation site of the pulpal floor was repaired with Mineral trioxide aggregate (MTA, ProRoot MTA, Dentsply Tulsa, Tulsa, OK, USA) under an operating microscope (OPMI, PICO, Carl Zeiss, Gottingen, Germany). Root canal treatment for tooth #36 was scheduled.
After 2 days, the patient presented with severe pain in the same tooth. After the access cavity was opened, unset MTA was found. Although all canals were negotiated and shaped, the tooth was symptomatic. The perforated site remained inflamed, even though it had been repaired with MTA. Based on the perforation size and contact period of Depulpin, the prognosis of the tooth was regarded as hopeless. Therefore, tooth #36 was extracted. After 4 days, the patient complained of pain in the left mandible even after the extraction. Distal septal bone loss around tooth #35 was observed, mobility was increased to degree 2, and sensibility of the tooth to the electric pulp test was absent. Pulp necrosis in tooth #35 was diagnosed and root canal treatment was initiated.
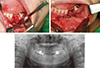
After 4 days, the patient reported persistent pain in the extraction site. Teeth #34 and 35 were sensitive to percussion, and pus discharge from the extraction site was observed. The patient was referred to the Department of Oral and Maxillofacial Surgery for evaluation of the extraction site. A bone scan showed increased inflammation of the left mandible. A biopsy was performed and the final diagnosis was determined as acute and chronic osteomyelitis of the left mandible. Extraction of teeth #33, 34, 35, and 37, and decortication and saucerization of the left mandible were performed (Figures 2a - 2c). After 3 months, the patient did not show any symptoms (Figure 2d).
A 39 year old woman was referred to the Department of Oral and Maxillofacial Surgery with acute pain and swelling in the left mandible. Nonsurgical endodontic treatment on tooth #35 and incision and drainage in the left mandible had been performed at a private clinic 1 week before. She experienced severe pain, left facial swelling, and restricted mouth opening after the treatment. She was admitted and treatment with intravenous antibiotics (Combicin Inj., 3 g, Samsung Pharm., Seoul, Korea) was scheduled. Her medical history was non-contributory.
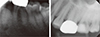
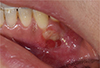
After 10 days, the patient was referred to the Department of Conservative Dentistry for root canal treatment of teeth #27 and 35. The patient exhibited a decrease in spontaneous pain in tooth #35 after administration of medication. A clinical examination revealed decreased left facial swelling and limited mouth opening. However, tenderness to palpation on gingival tissue around tooth #35 remained. A periapical radiolucent lesion on tooth #35 was observed and initiation of previous root canal treatment on tooth #27 was confirmed in the periapical radiograph (Figure 3). Previously initiated therapy with acute apical abscess on tooth #35, and previously initiated therapy with normal apical tissue on tooth #27 was diagnosed. Root canal treatments were scheduled for teeth #27 and 35. When temporary filling material was removed, it was observed that Depulpin had been used on tooth #27. Since root canal treatment of tooth #35 was initiated around the same time in the same local clinic, the use of Depulpin in tooth #35 was strongly suspected. After 1 month, teeth #33 and 34 demonstrated negative responses to an electric pulp test. Consequently, root canal treatments of teeth #33 and 34 were performed. After completion of root canal treatment of teeth #33 - 35, alveolar bone exposure persisted and the initial incision line of gingival tissue around #33 - 34 did not show signs of healing (Figure 4). The patient was referred to the Department of Oral and Maxillofacial Surgery for further evaluation. Additional radiographic examination was performed and a bone scan showed increased inflammation of the left mandible. Final diagnosis was determined as focal osteomyelitis of the mandible in the region around teeth #33 - 35. Teeth #33 and 34 were extracted, and decortication and saucerization of the left mandible were performed in the Department of Oral and Maxillofacial Surgery (Figures 5a and 5b). After surgery, the patient did not present with any specific symptoms (Figure 5c).
Depulpin was commonly used to provide quick pain relief by devitalization of pulp when effective anesthesia was not achieved. Depulpin contains 49% paraformaldehyde, 38% lidocaine, 5% clove oil, 3% Prussian balsam, and 2% chlorothymol. Paraformaldehyde causes devitalization of vital pulp and lidocaine produces anesthesia. Among other formaldehyde-containing pastes, formocresol is a well-known medicament used for the pulpotomy of primary teeth and has excellent tissue fixation activity. Although several reports have supported successful clinical outcomes with formocresol,56 the safety of formocresol has not been verified and frequently discussed. The use of formocresol is associated with cytotoxicity, allergenicity, mutagenicity, carcinogenicity, and teratogenic effects on animals.7 In addition, a study by Nishimura et al. reported that the use of formocresol caused chromosomal damage to human dental pulp cells.8 A study by Cambruzzi and Greenfeld revealed that excessive use of formocresol during root canal treatment caused bone necrosis.9 A study by Kawakam et al. reported tooth exfoliation and bone necrosis caused by formocresol leakage during root canal treatment.10
The major pharmacological effects of formocresol are derived from formaldehyde. Formaldehyde is the depolymerization product of paraformaldehyde. Paraformaldehyde fixes tissue by cross-linking the proteins, primarily between the residues of the basic amino acid lysine. It was reported that exposure to formaldehyde may irritate the eyes and upper respiratory tract.1112 High concentrations of formaldehyde may cause nasal obstruction, pulmonary edema, choking, dyspnea, and chest tightness.1314 A study by Tagger and Tagger compared pulpal and periapical effects of zinc oxide eugenol cement and paraformaldehyde in monkeys. When paraformaldehyde was applied, pulp necrosis and slight chronic periapical reaction were observed.15
Depulpin contains 2.5 times more paraformaldehyde than formocresol. Therefore, it is more cytotoxic than formocresol. A study by Moon et al. compared the effects of formocresol on pulpal and periapical tissues with those of Depulpin in rat teeth. Severe root resorption and necrosis of periapical tissue was observed in the Depulpin group and the study reported that Depulpin was more cytotoxic to dental pulp and periapical tissue than formocresol.16 In addition, a study by Hülsmann et al. reported that the marginal leakage of temporary filling material and iatrogenic perforation of the pulp chamber floor may result in diffusion of toxic intracanal medicaments (Toxavit, Lege artis, Dettenhausen, Germany) into the periodontal tissues.17
In the first case, the clinician missed the root canal orifice and applied Depulpin on the perforation site misjudged as a root canal orifice. Furthermore, the mesial wall of tooth #36 was not sealed. Hence, Depulpin was in direct contact with the alveolar bone or soft tissue. The patient presented with severe pain in the left mandible after extraction of tooth #36. Gradual necrosis of the left mandible followed and osteomyelitis occurred.
In the second case, application of Depulpin on tooth #27 was observed and a similar application on tooth #35 was strongly suspected. The patient presented with gradual loss of sensibility on teeth #33 and 34, gingival necrosis, and persistence of bone exposure around tooth #33. Final diagnosis was focal osteomyelitis of the mandible in the teeth #33 - 35 region, and decortication, saucerization, and extraction of teeth #33 and 34 were performed. Tooth #35 had a disto-occlusal cavity and the radiolucency of the apical area in an initial periapical radiograph provided evidence for pulp necrosis. If the medicament was applied in the necrotic pulp, it would have rapidly diffused and the paraformaldehyde-containing medicament may have leaked into the periodontium because of an inadequate temporary restoration.
A study by Ozgöz et al. reported gingival necrosis caused by paraformaldehyde-containing paste used on tooth #16 during root canal treatment.3 A study by Stabholz and Blush and another study by Di Felice and Lombardi reported necrotic bone and gingiva resulted from paraformaldehyde-containing paste (Toxavit) used during root canal treatment.1819
These case reports demonstrate unfavorable adverse effects of paraformaldehyde-containing paste on the periodontium and bone when used as a devitalizing agent during root canal treatment. We concluded that the use of paraformaldehyde-containing agents for devitalization of the inflamed pulp during root canal treatment is no longer indicated.
Dentists often face difficulties when there is failure of anesthesia in teeth diagnosed with irreversible pulpitis. Despite of its clinical benefit, use of paraformaldehyde-containing paste in such circumstances may lead to many noxious effects on the host tissue, such as periodontal destruction and bone necrosis. In the cases described here, injudicious use of paraformaldehyde-containing paste resulted in severe complications, such as osteomyelitis of the left mandible. Therefore, use of these medicaments must be restricted and clinicians should place emphasis on proper anesthetic management, access opening, pulp extirpation, and cleaning and shaping of root canals.
Figures and Tables
Figure 1
At initial visit, the patient complained of spontaneous throbbing pain in tooth #36. (a) Initial periapical radiograph; (b) Contaminated cotton pellets and paper points inserted in root canals; (c) Occlusal view under microscope. Perforation of the pulpal floor was observed; (d) Preoperative panoramic radiograph film. Arrow, perforation site; Asterisk, original canal.
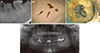
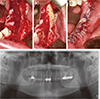
Figure 2
Clinical photographs during the surgical procedure. (a) Flap elevation and extraction of teeth #33, 34, 35, and 37; (b) Decortication and saucerization; (c) Suture; (d) Panoramic radiograph film after surgical procedure.
References
1. Cohen HP, Cha BY, Spångberg LS. Endodontic anesthesia in mandibular molars: a clinical study. J Endod. 1993; 19:370–373.

2. Kunisada M, Adachi A, Asano H, Horikawa T. Anaphylaxis due to formaldehyde released from root-canal disinfectant. Contact Dermatitis. 2002; 47:215–218.

3. Ozgöz M, Yagiz H, Ciçek Y, Tezel A. Gingival necrosis following the use of a paraformaldehyde-containing paste: a case report. Int Endod J. 2004; 37:157–161.

4. Tortorici S, Burruano F, Difalco P. Maxillary bone necrosis following the use of formaldehyde containing paste: management and case series. Br Dent J. 2007; 203:511–512.

5. Wesley DJ, Marshall FJ, Rosen S. The quantitation of formocresol as a root canal medicament. Oral Surg Oral Med Oral Pathol. 1970; 29:603–612.

6. Fuks AB, Bimstein E. Clinical evaluation of diluted formocresol pulpotomies in primary teeth of school children. Pediatr Dent. 1981; 3:321–324.
7. Lewis BB, Chestner SB. Formaldehyde in dentistry: a review of mutagenic and carcinogenic potential. J Am Dent Assoc. 1981; 103:429–434.

8. Nishimura H, Higo Y, Ohno M, Tsutsui TW, Tsutsui T. Ability of root canal antiseptics used in dental practice to induce chromosome aberrations in human dental pulp cells. Mutat Res. 2008; 649:45–53.

9. Cambruzzi JV, Greenfeld RS. Necrosis of crestal bone related to the use of excessive formocresol medication during endodontic treatment. J Endod. 1983; 9:565–567.

10. Kawakami J, Muto T, Shigeo K, Takeda S, Kanazawa M. Tooth exfoliation and necrosis of the crestal bone caused by the use of formocresol. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95:736–738.

11. Weber-Tschopp A, Fischer T, Grandjean E. Irritating effects of formaldehyde on man (author's transl). Int Arch Occup Environ Health. 1977; 39:207–218.
12. Sauder LR, Green DJ, Chatham MD, Kulle TJ. Acute pulmonary response of asthmatics to 3.0 ppm formaldehyde. Toxicol Ind Health. 1987; 3:569–578.

13. Porter JA. Letter: acute respiratory distress following formalin inhalation. Lancet. 1975; 2:603–604.
14. Solomons K, Cochrane JW. Formaldehyde toxicity. Part I. Occupational exposure and a report of 5 cases. S Afr Med J. 1984; 66:101–102.
15. Tagger E, Tagger M. Pulpal and periapical reactions to glutaraldehyde and paraformaldehyde pulpotomy dressing in monkeys. J Endod. 1984; 10:364–371.

16. Moon HI, Kim SH, Hwang YC, Oh BJ, Hwang IN, Kim SH, Jeong SW, Youn C, Oh WM. Pulpal and periapical reaction to formocresol and depulpin in the rat teeth. J Korean Acad Conserv Dent. 2002; 27:355–362.

17. Hülsmann M, Hornecker E, Redeker M. Periodontal destruction and tooth loss following pulp devitalization with Toxavit: report of a case. Dent Traumatol. 1993; 9:216–221.

18. Stabholz A, Blush MS. Necrosis of the crestal bone caused by the use of Toxavit. J Endod. 1983; 9:110–113.

19. Di Felice R, Lombardi T. Gingival and mandibular bone necrosis caused by a paraformaldehyde-containing paste. Endod Dent Traumatol. 1998; 14:196–198.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download