Abstract
Objectives
The purpose of the present study was to evaluate the effects of proanthocyanidin (PAC), a crosslinking agent, on the physical properties of a collagen hydrogel and the behavior of human periodontal ligament cells (hPDLCs) cultured in the scaffold.
Materials and Methods
Viability of hPDLCs treated with PAC was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The physical properties of PAC treated collagen hydrogel scaffold were evaluated by the measurement of setting time, surface roughness, and differential scanning calorimetry (DSC). The behavior of the hPDLCs in the collagen scaffold was evaluated by cell morphology observation and cell numbers counting.
Results
The setting time of the collagen scaffold was shortened in the presence of PAC (p < 0.05). The surface roughness of the PAC-treated collagen was higher compared to the untreated control group (p < 0.05). The thermogram of the crosslinked collagen exhibited a higher endothermic peak compared to the uncrosslinked one. Cells in the PAC-treated collagen were observed to attach in closer proximity to one another with more cytoplasmic extensions compared to cells in the untreated control group. The number of cells cultured in the PAC-treated collagen scaffolds was significantly increased compared to the untreated control (p < 0.05).
Conclusions
Our results showed that PAC enhanced the physical properties of the collagen scaffold. Furthermore, the proliferation of hPDLCs cultured in the collagen scaffold crosslinked with PAC was facilitated. Conclusively, the application of PAC to the collagen scaffold may be beneficial for engineering-based periodontal ligament regeneration in delayed replantation.
Tooth avulsion is a common but serious traumatic injury in the dental field. When a tooth is avulsed, the survival of the periodontal ligament (PDL) cells of the tooth is a decisive factor on the fate of the avulsed tooth. Improper storage or delayed replantation of the tooth in the alveolar socket results in the desiccation and necrosis of the periodontal cells. The success rate of the treatment of an avulsed tooth is reported as low, varying from 4 to 50%.1 In delayed replantation cases, dentoalveolar ankylosis or replacement resorption of the root is frequently observed.2 These events can cause loss of the tooth and create dentoalveolar deformities especially in the growth period. Currently, however, there is no reliable treatment option to prevent such unfavorable clinical condition.3 Dental implants are considered the preferred alternative treatment option in cases of tooth loss. However, implants can fail and do not have the ability to remodel with the surrounding bone due to the lack of PDL. For that reason, implants also cannot be recommended during the period of skeletal growth. Therefore, there is a strong demand for a new strategy for the treatment of delayed replantation.
With the development of tissue engineering and regenerative medicine, there have been efforts to regenerate and reconstruct missing periodontal tissues using cell-based therapy. It has been known that PDL cells have the characteristics of stem cells that can differentiate into the components of the periodontium.45678910 PDL cells can also induce the formation of new periodontal tissue when applied into the periodontal defect area under certain conditions.11 It was reported that the delayed replanted tooth with the application of cultured PDL cells or collagen coating with certain bioactive molecules exhibited the re-establishment of PDL-like tissue in animals.512 The clinical approach with periodontal tissue regeneration in delayed replantation is being translated from the possibility into the reality.
In tissue engineering, there are three main components, including stem cells, bioactive molecules, and scaffolds. Among these, scaffolds play a crucial role in tissue engineering by mimicking the function of the extracellular matrix (ECM), and by offering a three-dimensional spaciotemporal microenvironment for cell attachment, migration, proliferation, differentiation, and metabolism1314 A suitable scaffold also has other characteristics including a highly porous interconnected network with proper surface properties, biocompatibility, and biodegradability harmonious with the rate of cell/tissue growth and maturation.1115 Collagen, a significant constituent of the natural ECM, has been regarded as the ideal material for soft tissue engineering constructs due to its excellent biocompatibility and biodegradability.161718 However, collagen-based scaffolds have a crucial limitation on the use of artificial substitutes because of its high degradation rate and poor mechanical properties.161718 Therefore, the crosslinking of collagen is an effective strategy to modify the drawbacks and provide strength, reinforcement, and stabilization to the fibrils enough to be used as biomaterials.1719 There are physical and chemical crosslinking methods currently available. Despite its excellent biocompatibility, physical methods, including the use of dehydrothermal treatments or ultraviolet irradiation, do not currently exhibit a high enough degree of crosslinking in tissue engineering. As a result, chemical methods, including the use of aldehydes, isocyanates, or carbodiimides, are still widely used. Among the chemical agents, glutaraldehyde is the most commonly available traditional agent. However, there are major issues such as excessive crosslinking and high cytotoxicity that limit the clinical applicability of these agents.142021 Therefore, it is necessary to discover new crosslinking reagents with lower or no toxicity.
Proanthocyanidins (PACs) are naturally occurring polyphenolic bioflavonoids that are diverse and widely available in fruits, vegetables, seeds, nuts, flowers, and bark.22 These compounds possess a broad spectrum of biological and pharmacological properties against free radicals and oxidative stress as antioxidants.23 They are also known as natural biocompatible collagen crosslinkers and biomodification agents of dentin due to their ability to increase collagen synthesis.21 It was reported that PACs crosslinked the recombinant human collagen-peptide-chitosan scaffold, promoted cell proliferation on the scaffold surface, and permitted cell migration into the scaffold.17 To date, however, there has been no research on the effects of PACs, a crosslinking agent, on human periodontal ligament cells (hPDLCs) cultured in the collagen hydrogel scaffold. This study aimed to investigate the effects of PACs on the collagen hydrogel scaffold in terms of changes in physical properties of the collagen hydrogel scaffold and behavior of hPDLCs cultured in the scaffold.
Human periodontal ligament tissues obtained from freshly extracted teeth were removed aseptically, rinsed with phosphate buffered saline solution (PBS, HyClone Laboratories, Logan, UT, USA), and placed in a 60 mm dish (Nunc, Roskilde, Denmark). The tissues were then minced with a blade into small fragments and cultured in minimal essential medium-α (MEM-α, HyClone Laboratories) containing 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA) along with 100 U/mL penicillin and 100 U/mL streptomycin (Invitrogen). The cultures were maintained at 37℃ in a humidified atmosphere of 5% CO2 and 95% air. Cell cultures between the third and fifth passages were used in this study. All experimental procedures were approved by the Institutional Review Board (IRB No. CUH 2013-11-014) of the Chonbuk National University Hospital (Jeonju, Korea).
Cells were seeded in 24 well culture plates at a density of 2 × 104 cells per well and incubated for 24 hours to allow for initial attachment. The cells were then treated with various concentrations of PAC (Sigma-Aldrich, St. Louis, MO, USA) for up to 7 days. Cell viability was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, 200 mL MTT solution (0.5 mg/mL in PBS) was added to each well and incubated for 2 hours. Subsequently, 200 mL dimethyl sulfoxide (DMSO, Amresco, Solon, OH, USA) was added to each well. The plates were then shaken until the crystals had dissolved, and the solution in each well was transferred to a 96 well tissue culture plate. Reduced MTT was then measured spectrophotometrically at 540 nm in a microplate reader (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany).
Purified bovine collagen solution (PurCol, Advanced BioMatrix Inc., Tucson, AZ, USA) was mixed with 10 × PBS in a ratio of 8:1 (volume). The pH was adjusted to 7.4 by adding sodium hydroxide solution (Bio Basic Canada Inc., Markham, ON, Canada), and the mixture was treated with 0 or 1 µM of PAC.
The setting time of the collagen scaffold was measured using the tilting method. The collagen scaffold was treated with 0 or 1 µM of PAC, and inserted into an eppendorf tube. The hydrogel collagen was considered to be set if it did not flow when the tube was tilted to an angle of 45°. The samples (n = 3) were tested prior to their anticipated setting time and at 30 seconds intervals.
The collagen specimen (0.5 mm in diameter and 1.5 mm in height) was prepared and dried using a critical point dryer (Electronic Microscopy Sciences, Hatfield, PA, USA). The atomic force microscopy (AFM) apparatus (Bruker, Karlsruhe, Germany) was operated in a tapping mode using standard tapping probes (RTSEP Silicon AFM tips, Bruker). All the image analysis was carried out using Nanoscope analysis (ver. 1.4, Bruker). An area of 100 µm2 (10 µm × 10 µm) was captured for the AFM image using a scanning speed of 1 Hz.
DSC (Q20, TA Instruments, New Castle, DE, USA) was used to determine the thermal behavior of the crosslinked collagen scaffolds under wet conditions. The collagen scaffolds were heated at 10 ℃/min under nitrogen air at a temperature range from 30 to 330℃. The denaturation temperature was determined as the peak value of the corresponding endothermic phenomena, and the denaturation enthalpy was calculated as the area of the peak regarding the weight of collagen.
The collagen hydrogel scaffold (1.5 mL) was mixed with the cell suspension (1 × 105) and inserted into a silicon tube mold (10 mm in diameter and 2 mm in height). After 3 day incubation period in the presence of MEM-α (HyClone Laboratories) containing 10% FBS (Invitrogen), the samples were fixed with 2.5% glutaraldehyde (Sigma-Aldrich) for 2 hours. The samples were then dehydrated in increasing concentrations of ethanol (70, 80, 90, 95, and 100%) for 30 minutes at each concentration, immersed in n-butyl alcohol (Junsei Chemical Co., Tokyo, Japan) for 20 minutes, and dried using a critical point dryer (EMS850, Electronic Microscopy Sciences). Scanning electron microscopy (SEM) was performed using an SN-3000 system (Hitachi, Tokyo, Japan) operated at 10 kV.
1.5 mL of collagen solution containing PAC (0 or 1 µM) was mixed with the cell suspension (1 × 105), and the solution was transferred to six-well culture plates and gelated. The cells were then incubated in the presence of MEM-α (HyClone Laboratories) containing 10% FBS (Invitrogen). After 3 days, the cells cultured in half of the plates were stained with 4',6-diamidino-2-phenylindole (DAPI, Invitrogen). Then, cell numbers were determined under an optical (Leica, Solms, Germany) or fluorescence microscope (Carl Zeiss, Jena, Germany) by two calibrated examiners who did not have any information regarding the experiment (n = 5). Briefly, a rectangle was inscribed in a round six-well plate and divided into nine equal areas. Three images were then acquired from each area, and the number of cells (mean ± standard deviation [SD]) in each area was counted by the examiners.
To select the biologically effective concentration of PAC, we assessed the effect of PAC on cell viability using an MTT assay. As shown in Figure 1, the hPDLCs exposed to PAC concentrations of 0.1, 1, and 10 µM did not exhibit any difference compared to cells in the control group throughout the experimental period. However, the cells treated with a 20 µM concentration of PAC showed a significantly lower viability (p < 0.05). For this reason, we chose the concentration of 10 µM for the current study.
To assess the physical properties of crosslinked collagen scaffold, the setting time and surface roughness were measured. The setting time of the PAC-treated collagen (9 minutes ± 30 seconds) was significantly shorter than that of the untreated control (16 minutes ± 30 seconds) (p < 0.05, Figure 2). As shown in Figures 3a - 3c, the surface roughness of the PAC-treated collagen was significantly higher than that of the untreated collagen (p < 0.05).
DSC studies were undertaken to evaluate the thermal behavior of the collagen scaffolds. The thermograms are displayed in Figure 4. The thermogram of the uncrosslinked collagen exhibited an endothermic sharp peak at 77.35℃ due to its denaturation, while the collagen crosslinked with 1 µM PAC exhibited a peak at 113.49℃.
We assessed whether the crosslinking of collagen using PAC promotes the growth and attachment of PDLCs. SEM observation was performed to morphologically analyze the hPDLCs cultured in collagen with or without PAC. The cells in PAC-treated collagen were observed to spread across the substrate and attach in close proximity to one another with numerous cytoplasmic extensions, whereas the cells in the uncrosslinked collagen matrix did not exhibit any of these morphological characteristics (Figures 5a and 5b). We also determined the cell number under an optical (Figures 6a and 6b) and fluorescence microscope (Figures 6c and 6d). The cell number in the PAC-treated group was statistically higher than that in the untreated control group (p < 0.05, Figure 6e).
With the development of tissue engineering in the dental field, cell-based therapy is becoming the new approach for repairing and regenerating tooth supporting tissue.10 For this purpose, a collagen scaffold has been employed in several studies to regenerate the periodontal attachment apparatus.52425 In accordance with previous studies, PDL tissue regeneration can be a new strategy for the treatment of delayed replantation. Furthermore, the application of autologous PDLCs with the collagen scaffold can potentially aid in PDL tissue regeneration. However, enhancing the mechanical properties including the appropriate matrix degradation kinetics of the collagen scaffold can be a prerequisite for the use of the collagen scaffold in PDL tissue regeneration.
Crosslinking has become an important method to slow down the biodegradation rate and enhance the mechanical properties of the collagen scaffold.20 PACs, natural biocompatible collagen cross-linkers, are broadly distributed in the plant kingdom.26 Moreover, due to its high dentin bioactivity and ability to increase collagen synthesis, this bioactive resource has received great interest in the field of biomodification of dentin for enhancing the adhesive interface.1921 Since PACs exhibit a close affinity to collagen and dentin and have the ability to crosslink collagen, PAC treatment may result in synergistic and beneficial effects on PDL tissue regeneration in delayed replantation when considering the specific clinical circumstances osculating with the surface of dentin. In addition, PAC treatment into the collagen scaffold was reported to not only promote cell proliferation, but also permit cell migration into the scaffold.17 However, there have been no studies on the effects of PAC on hPDLCs cultured in the collagen scaffold.
First, we assessed the effect of PAC on cell viability using an MTT assay to select the optimal concentration of PAC for the current study. In this study, 10 µM of PAC did not show any cytotoxic effect throughout the experimental period, but the cells treated with a 20 µM of PAC showed significantly lower viability. Furthermore, many previous studies have agreed that 1 to 50 µM of PAC are effective concentrations for evaluating proliferation and differentiation of cells.272829 Therefore, in accordance with the previous studies and results of our study, we chose the concentration of 10 µM for the current study.
Then, we evaluated the effects of PAC on the physical properties of the collagen scaffold including setting time, surface roughness, and thermal behavior. The setting time of PAC-treated collagen was significantly reduced compared to the untreated collagen (p < 0.05, Figure 2). The gelation of the hydrogel is an important precondition as a scaffold, providing a more suitable environment for the inclusion and proliferation of cells in the gel.30 The reduction of the setting time can allow more opportunities for the cells to adhere to the scaffold. Due to the close-set population, each cell can transmit signals regarding growth and proliferation to other cells more efficiently and consequently grow faster. Furthermore, the surface roughness of the PAC-treated collagen scaffold increased compared to the untreated control (p < 0.05, Figure 3). It is generally believed that the mechanical stimulus such as the rough surface of the substrate could improve cellular behavior on contact with scaffolds for tissue engineering.83031 In this respect, increased surface roughness of the collagen scaffold may influence the proliferation of hPDLCs. DSC was used to investigate the effect of PAC crosslinking on collagen denaturation. At the denaturation temperature (TD), the helix-to-coil transition occurred due to the rupture of the hydrogen bonds in collagen.30 In this study, the TD was affected by the PAC treatment. The PAC-treated collagen exhibited a sharp endothermic peak at a higher denaturation point compared to the untreated collagen (Figure 4). Denaturation enthalpy (ΔH), calculated as the area under the peak, may be related to the crosslink density.30 The PAC-treated collagen required more energy for denaturation compared to the untreated collagen sample, indicating the PAC treatment into the collagen scaffolds leads to stabilization of the substrate.
The effects of PACs on the proliferation through morphological observations and cell number counting of hPDLCs cultured in the collagen scaffold were then evaluated. In the morphological analysis, the cells in the PAC-treated collagen were spread across the scaffold and attached in close proximity with one another with numerous cytoplasmic extensions while the cells in the untreated collagen were not (Figure 5). The cell number in the PAC-treated collagen was statistically higher than in the untreated collagen, regardless of the type of microscope, indicating that PAC treatment with the collagen scaffold promoted the proliferation of hPDLCs (Figure 6). The increased proliferation of hPDLCs may be due to the enhanced physical properties demonstrated in our current study. Furthermore, these findings are supported by several previous studies which showed that the crosslinking of collagen leads to an improvement in cell adhesion and proliferation.1718203233 When counting cells, DAPI staining was used to help distinguish cells from the collagen substrate. Consequently, the cell number was double-checked using both optical and fluorescence microscopy. Overall, the PAC-treated collagen scaffold exhibited enhanced physical characteristics including quicker setting time, increased surface roughness, and a higher denaturation point. In addition, proliferation of hPDLCs was increased in the PAC-treated collagen scaffold.
Within the limitations of this study, the results proved that crosslinking of collagen with PAC treatment could improve the physical properties of the collagen scaffold and enhance the proliferation of hPDLCs. Based on these results, improvements in the physical properties of the collagen scaffold using PAC treatment should positively influence the behavior and fate of cells including cell survival, attachment, and proliferation. These findings offer a step forward in the study of collagen-based periodontal tissue engineering for delayed replantation.
Figures and Tables
 | Figure 1Effects of PAC on cell viability measured by the MTT assay. *Significant difference compared with the control (p < 0.05). MTT assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay; OD, optical density; PAC, proanthocyanidin. |
 | Figure 2Setting times of uncrosslinked and crosslinked collagen as determined by the tilting method. *Significant difference compared with the control (p < 0.05). CON, control; PAC, proanthocyanidin. |
 | Figure 3Effect of PAC on the surface roughness of the collagen scaffolds. AFM images of (a) untreated collagen and (b) PAC-treated collagen. (c) Quantitative evaluation of the surface roughness. *Significant difference compared with control (p < 0.05). CON, control; PAC, proanthocyanidin; AFM, atomic force microscopy. |
 | Figure 4Differential scanning calorimetric curves of uncrosslinked and crosslinked collagen scaffolds. PAC, proanthocyanidin; TD, denaturation temperature; ΔH, denaturation enthalpy. |
 | Figure 5SEM images of hPDLCs incubated on (a) uncrosslinked and (b) crosslinked collagen (×1,000) for three days. |
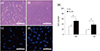 | Figure 6Proliferation analyses of hPDLCs cultured in the collagen scaffolds. Optical microscope images of hPDLCs cultured in (a) untreated and (b) PAC-treated collagen matrices (×200). Representative fluorescence microscopy images (×400) showing hPDLCs (stained with DAPI) growing in the collagen scaffolds after three days of culture in the (c) absence or (d) presence of PAC. (e) Effects of PAC-induced crosslinking on the proliferation of hPDLCs cultured in collagen. *Significant difference compared with control (p < 0.05). hPDLC, human periodontal ligament cells; OM, optical microscope; CM, confocal laser scanning microscope; COL, collagen without PAC; COL/PAC, collagen treated with PAC, PAC, proanthocyanidin. |
Acknowledgement
This paper was supported by the Biomedical Research Institute of the Chonbuk National University Hospital in 2015.
References
1. Krasner P, Rankow HJ. New philosophy for the treatment of avulsed teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 79:616–623.

2. Hermann NV, Lauridsen E, Ahrensburg SS, Gerds TA, Andreasen JO. Periodontal healing complications following extrusive and lateral luxation in the permanent dentition: a longitudinal cohort study. Dent Traumatol. 2012; 28:394–402.

3. Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. Replantation of 400 avulsed permanent incisors. 4. Factors related to periodontal ligament healing. Endod Dent Traumatol. 1995; 11:76–89.

4. Mattioli-Belmonte M, Teti G, Salvatore V, Focaroli S, Orciani M, Dicarlo M, Fini M, Orsini G, Di Primio R, Falconi M. Stem cell origin differently affects bone tissue engineering strategies. Front Physiol. 2015; 6:266.

5. Wang Y, Cheung GS, Xu X, Zhao S, Zhang C. The effect of cultured autologous periodontal ligament cells on the healing of delayed autotransplanted dog's teeth. J Endod. 2010; 36:264–267.

6. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.

7. Lekic P, Rojas J, Birek C, Tenenbaum H, McCulloch CA. Phenotypic comparison of periodontal ligament cells in vivo and in vitro. J Periodont Res. 2001; 36:71–79.

8. Yang ZH, Zhang XJ, Dang NN, Ma ZF, Xu L, Wu JJ, Sun YJ, Duan YZ, Lin Z, Jin Y. Apical tooth germ cell-conditioned medium enhances the differentiation of periodontal ligament stem cells into cementum/periodontal ligament-like tissues. J Periodontal Res. 2009; 44:199–210.

9. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009; 88:792–806.

10. Catón J, Bostanci N, Remboutsika E, De Bari C, Mitsiadis TA. Future dentistry: cell therapy meets tooth and periodontal repair and regeneration. J Cell Mol Med. 2011; 15:1054–1065.

11. Han J, Menicanin D, Gronthos S, Bartold PM. Stem cells, tissue engineering and periodontal regeneration. Aust Dent J. 2014; 59:Supplement 1. 117–130.

12. Zhou Y, Li Y, Mao L, Peng H. Periodontal healing by periodontal ligament cell sheets in a teeth replantation model. Arch Oral Biol. 2012; 57:169–176.

13. Ivanovski S, Vaquette C, Gronthos S, Hutmacher DW, Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res. 2014; 93:1212–1221.

14. Davidenko N, Schuster CF, Bax DV, Raynal N, Farndale RW, Best SM, Cameron RE. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015; 25:131–142.

15. Hutmacher DW, Cool S. Concepts of scaffold-based tissue engineering: the rationale to use solid free-form fabrication techniques. J Cell Mol Med. 2007; 11:654–669.

16. Wang Y, Van Manh N, Wang H, Zhong X, Zhang X, Li C. Synergistic intrafibrillar/extrafibrillar mineralization of collagen scaffolds based on a biomimetic strategy to promote the regeneration of bone defects. Int J Nanomedicine. 2016; 11:2053–2067.
17. Zhang J, Deng A, Yang Y, Gao L, Xu N, Liu X, Hu L, Chen J, Yang S. HPLC detection of loss rate and cell migration of HUVECs in a proanthocyanidin cross-linked recombinant human collagen-peptide (RHC)-chitosan scaffold. Mater Sci Eng C Mater Biol Appl. 2015; 56:555–563.

18. Fortunati D, Chau DY, Wang Z, Collighan RJ, Griffin M. Cross-linking of collagen I by tissue transglutaminase provides a promising biomaterial for promoting bone healing. Amino Acids. 2014; 46:1751–1761.

19. Castellan CS, Bedran-Russo AK, Antunes A, Pereira PN. Effect of dentin biomodification using naturally derived collagen cross-linkers: one-year bond strength study. Int J Dent. 2013; 2013:918010.

20. Ma L, Gao C, Mao Z, Zhou J, Shen J. Enhanced biological stability of collagen porous scaffolds by using amino acids as novel cross-linking bridges. Biomaterials. 2004; 25:2997–3004.

21. Bedran-Russo AK, Pauli GF, Chen SN, McAlpine J, Castellan CS, Phansalkar RS, Aguiar TR, Vidal CM, Napotilano JG, Nam JW, Leme AA. Dentin biomodification: strategies, renewable resources and clinical applications. Dent Mater. 2014; 30:62–76.

22. Bagchi D, Bagchi M, Stohs Sj, Ray SD, Sen CK, Preuss HG. Cellular protection with proanthocyanidins derived from grape seeds. Ann N Y Acad Sci. 2002; 957:260–270.

23. Ye X, Krohn RL, Liu W, Joshi SS, Kuszynski CA, McGinn TR, Bagchi M, Preuss HG, Stohs SJ, Bagchi D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999; 196:99–108.

24. Kato A, Miyaji H, Ishizuka R, Tokunaga K, Inoue K, Kosen Y, Yokoyama H, Sugaya T, Tanaka S, Sakagami R, Kawanami M. Combination of root surface modification with BMP-2 and collagen hydrogel scaffold implantation for periodontal healing in beagle dogs. Open Dent J. 2015; 9:52–59.

25. Zhu W, Zhang Q, Zhang Y, Cen L, Wang J. PDL regeneration via cell homing in delayed replantation of avulsed teeth. J Transl Med. 2015; 13:357.

26. Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003; 65:118–124.

27. Deters A, Dauer A, Schnetz E, Fartasch M, Hensel A. High molecular compounds (polysaccharides and proanthocyanidins) from Hamamelis virginiana bark: influence on human skin keratinocyte proliferation and differentiation and influence on irritated skin. Phytochemistry. 2001; 58:949–958.

28. Neuwirt H, Arias MC, Puhr M, Hobisch A, Culig Z. Oligomeric proanthocyanidin complexes (OPC) exert anti-proliferative and pro-apoptotic effects on prostate cancer cells. Prostate. 2008; 68:1647–1654.

29. Xie ZY, Wu BH, Yang ZG, Chen XF, Chen QS. Differentiation of human promyelocytic leukemia HL-60 cells induced by proanthocyanidin and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013; 21:920–925.
30. Madaghiele M, Calò E, Salvatore L, Bonfrate V, Pedone D, Frigione M, Sannino A. Assessment of collagen crosslinking and denaturation for the design of regenerative scaffolds. J Biomed Mater Res A. 2016; 104:186–194.

31. Faia-Torres AB, Guimond-Lischer S, Rottmar M, Charnley M, Goren T, Maniura-Weber K, Spencer ND, Reis RL, Textor M, Neves NM. Differential regulation of osteogenic differentiation of stem cells on surface roughness gradients. Biomaterials. 2014; 35:9023–9032.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download