Abstract
Objectives
The purpose of this study was to investigate the involvement of TRPA1 in the cinnamaldehyde-induced pulpal blood flow (PBF) change in the feline dental pulp.
Materials and Methods
Mandibles of eight cats were immobilized and PBF was monitored with a laser Doppler flowmetry at the mandibular canine tooth. To evaluate the effect of cinnamaldehyde on PBF, cinnamaldehyde was injected into the pulp through the lingual artery at a constant rate for 60 seconds. As a control, a mixture of 70% ethanol and 30% dimethyl sulfoxide (DMSO, vehicle) was used. To evaluate the involvement of transient receptor potential ankyrin 1 (TRPA1) in PBF change, AP18, a specific TRPA1 antagonist, was applied into the pulp through the Class V dentinal cavity followed by cinnamaldehyde-administration 3 minutes later. The paired variables of experimental data were statistically analyzed using paired t-test. A p value of less than 0.05 was considered as statistically significant.
Results
Administration of cinnamaldehyde (0.5 mg/kg, intra-arterial [i.a.]) induced significant increases in PBF (p < 0.05). While administration of a TRPA1 antagonist, AP18 (2.5 - 3.0 mM, into the dentinal cavity [i.c.]) caused insignificant change of PBF (p > 0.05), administration of cinnamaldehyde (0.5 mg/kg, i.a.) following the application of AP18 (2.5 - 3.0 mM, i.c.) resulted in an attenuation of PBF increase from the control level (p < 0.05). As a result, a TRPA1 antagonist, AP18 effectively inhibited the vasodilative effect of cinnamaldehyde (p < 0.05).
A primary sensory nerve fiber is activated by a variety of stimuli and subsequently releases neuropeptides, which induce vasodilatation, extravasation of protein, and recruitment/regulation of immune cells which are neutrophils, macrophages, lymphocytes and mast cells. This phenomenon is termed neurogenic inflammation.1 Vasodilatation is the first vascular reactions during neurogenic inflammation in the dental pulp.2
The transient receptor potential ankyrin 1 (TRPA1) is a non-selective calcium permeable cationic channel belonging to the TRP channel superfamily.3 TRPA1 is highly expressed in sensory neurons which have emerged as an important molecular target for several types of pain in dorsal root ganglia and trigeminal ganglia, where it colocalizes with another TRP channel, TRP Vanilloid receptor 1 (TRPV1).45 In human teeth, TRPA1 is highly expressed in dental pulp fibroblasts where it might be involved in cold responses and pain associated with mechanotransduction6 and the majority of pulpal afferents also express TRPA1.7 Radresa et al. reported that treatment of TRPA1 antagonist inhibits signs of hypersensitivity in many rodent model study about inflammatory pain.8 Therefore, TRPA1 might be considered an important target for the management of dental sensitivity.
TRPA1 acts as a cellular sensor for a variety of irritants. Initially reported to sense noxious cold,9 TRPA1 has subsequently been reported to be stimulated by some divalent ions (Ca2+, Zn2+), and by a number of exogenous electrophilic compounds, such as the pungent ingredients in mustard oil (allyl isothiocyanate), ginger and cinnamon oil (cinnamaldehyde),1011 all of which can induce nocifensive behaviors, burning pain sensation, and sensory neuron sensitization in animals and man.8 Cinnamaldehyde is one of the main constituents of cinnamon and an aromatic aldehyde which has been reported to have multiple potential therapeutic activities.12 It has been used as an ingredient of tooth pastes and to control toothache, oral microbiota and to treat halitosis.1314
The previous studies have shown that both cinnamaldehyde and TRPA1 may be involved in the control of blood flow in skin15 and heart of rat.12 Despite oral use of cinnamon, functional relation between cinnamaldehyde and dental pulp circulation remains to be clarified. The purpose of this study was to investigate the involvement of TRPA1 in the cinnamaldehyde-induced pulpal blood flow (PBF) change in the feline dental pulp using laser Doppler flowmetry in order to elucidate neurogenic inflammation in the dental pulp.
All procedures that involved the use of animals were approved by the Institutional Care and Use Committee of the School of Dentistry, Kyungpook National University. The experiments were conducted with eight cats weighing 2.2 - 3.2 kg. Cats were anesthetized with a mixture of ketamine (75 mg/kg) and acepromazine (2.5 mg/kg) by intramuscular injection. Supplemental anesthetics, which were a mixture of alpha-chloralose (40 mg/kg) and urethane (500 mg/kg), were injected through the femoral vein as needed to maintain the appropriate level of anesthesia, assessed by the absence of changes in systemic blood pressure (SBP) or nociceptive reflexes to noxious stimuli. Periapical radiographs of canine teeth were taken to ensure the maturity of apices and to evaluate the size of the pulp. To maintain the airway, tracheostomy was carried out with an endotracheal tube. Femoral vein was cannulated to inject supplemental anesthetics and femoral artery was cannulated to monitor SBP with a pressure transducer (Figure 1).
Both jaws were immobilized by intermaxillary splinting with dental plaster and a steel rod that was anchored to the base of experimental table with a locking device. Body temperature was kept at 37.0 ± 0.5℃ with a heating pad and monitored with a rectal thermometer.
Lingual artery was cannulated to administrate cinnamaldehyde into the dental pulp. For the administration of drugs to the dental pulp, a class V dentinal cavity (1.5 mm in width, 2 mm in length and 0.5 mm in depth) was prepared on the middle third of the labial surface of the crown with a high speed #701 tapered fissure bur. The exposed dentin surface was etched with 32% phosphoric acid for 10 seconds so that smear layer was removed and the dentinal tubules were open.1617
Cinnamaldehyde (Sigma Chemical, St. Louis, MO, USA) was dissolved in a mixture of 70% ethanol and 30% dimethyl sulfoxide (DMSO) and diluted to make stock solutions of 10 mg/mL concentration. A solution of cinnamaldehyde at the desired concentration was prepared daily by dilution of the stock solution with the mixture of 70% ethanol and 30% DMSO. In each experiment, cinnamaldehyde was titrated to exert maximum effect on PBF without influencing SBP to minimize or eliminate systemic effect. All injections and measurements were started 60 minutes after the preparation of animal for a stable hemodynamic condition. Earlier studies have reported that a minimum of 30 to 50 minutes is required after cavity preparation for PBF to return to the control level.17 In order to evaluate the effect of cinnamaldehyde on PBF, 2 mL of 0.5 mg/kg cinnamaldehyde was injected through the lingual artery at a constant rate for 60 seconds, followed by a flush of 0.3 mL isotonic saline. As a control, 2 mL of cinnamaldehyde vehicle (a mixture of 70% ethanol and 30% DMSO) was injected before the administration of cinnamaldehyde.
A specific TRPA1 antagonist, AP18 (Sigma Chemical) was dissolved in 100% DMSO and diluted to make stock solutions of 1 mg/mL concentration. A solution of AP18 at the desired concentration was prepared by dilution of the stock solution with DMSO. AP18 was titrated to exert maximum inhibitory effect on cinnamaldehyde-induced PBF change without influencing PBF. 1 µL of AP18 (2.5 - 3.0 mM) was placed into the dentinal cavity (i.c.) followed by intra-arterial (i.a.) administration of cinnamaldehyde three minutes later. The cavity was flushed with isotonic saline for the following experiment.
Change in PBF was measured by using a laser Doppler flowmeter (PeriFlux 4001, Perimed, Stockholm, Sweden). A shallow cavity was prepared at the cervical third of the labial surface of canine under copious running water to expose the dentin using a high-speed inverted cone bur. It was placed approximately 3 mm from the marginal gingiva to eliminate the influence of the gingival blood flow. A laser Doppler flowmeter probe (PF416, Perimed) was positioned at right angles and 0.1 - 0.2 mm away from the axial wall of the prepared cavity using a micromanipulator (MMN-3, Narishige, Tokyo, Japan). To avoid drying of the dentin, isotonic saline was flooded between the dentin surface and the probe tip. PBF was monitored continuously and recorded with a computer software (LabScribe2, iWorx Systems Inc., Dover, NH, USA). Control level of PBF was established by recording it for three minutes prior to each drug. To determine the effect of drug, the maximum change of PBF was evaluated.
In order to evaluate the effect of a TRPA1 antagonist, AP18, on cinnamaldehyde-induced PBF change, cinnamaldehyde was administered three minutes after application of AP18 and then PBF was recorded and compared with the control level.
All numerical data in the text were expressed as percent change from control and mean ± standard error of the mean (SEM). The paired variables of control and experimental data were statistically analyzed using paired t-test. A p value of less than 0.05 was considered as statistically significant.
Change in PBF in response to cinnamaldehyde is presented in Figure 2 and Table 1. Application of cinnamaldehyde (0.5 mg/kg, i.a.) showed significant increase in PBF (p < 0.05). After cinnamaldehyde administration, the mean peak values of PBF were increased to 88.02 ± 11.56% (n = 19) from the control level. Administration of cinnamaldehyde vehicle, a mixture of 70% ethanol and 30% DMSO, itself did not cause any significant change of PBF.
Typical strip-chart recordings of SBP and PBF in response to cinnamaldehyde with vehicle and cinnamaldehyde with TRPA1 antagonist are presented in Figure 2. SBP remained unchanged both during and after the periods of drug administration.
Administration of cinnamaldehyde (0.5 mg/kg, i.a.) following the application of a TRPA1 antagonist, AP18 (2.5 - 3.0 mM, i.c.) resulted in an increase of PBF by 21.25 ± 3.80% (n = 19) from the control (p < 0.05), whereas the antagonist itself did not cause any significant change of PBF (Table 2).
In the present study, PBF was increased significantly when cinnamaldehyde was injected into the dental pulp through the lingual artery. TRPA1 antagonist AP18 was shown to effectively inhibit cinnamaldehyde-induced vasodilation. These findings strongly show the evidence that TRPA1 stimulated by cinnamaldehyde is involved in the mediation of vasodilation in dental pulp.
TRPA1 channels localized in primary afferent neurons may play a crucial role in the vascular reaction of neurogenic inflammation. It has been proposed that TRPA1 promotes inflammation through its mediation in not only neural (direct) but also immune cell (indirect) activation.18 TRPA1 activation create increased neuronal activity that induces the release of various neuropeptides and neurotransmitters, such as neurokinin A, substance P, and calcitonin gene-related peptide (CGRP). These materials lead to vasodilation and gather immune cells to the site.19 These immune cells will finally secrete a variety of signaling molecules, including the TRPA1 agonists hypochlorite from neutrophils, hydrogen peroxide from granulocytes, and prostaglandins from mast cells, macrophages and dendritic cells that will reactivate the neuron.20 The tissue damage often accompanies TRPA1 stimulation and produces reactive oxygen species followed by induction of liposome peroxidation and the generation of additional TRPA1 agonists including 4-oxo-2-nonenal and 4-hydroxynonenal.2122 Therefore, TRPA1 plays a role as a key mediator of the feed forward loop that allows local inflammation to maintain.
The vasodilatory effect of cinnamaldehyde has been investigated in previous studies. Pozsgai et al. investigated TRPA1-induced responses in the vasculature in response to TRPA1 agonists (allyl isothiocyanate and cinnamaldehyde) using wild-type (WT) and TRPA1 knockout (KO) mice.23 Cinnamaldehyde triggered a significant increase in the hind paw blood flow in WT, but not in TRPA1 KO mice.23 Cinnamaldehyde also induced dose-dependent relaxation in WT and TRPA1 KO mesenteric arterial rings in vitro and this relaxation was significantly less potent in TRPA1 KO compared with WT arteries, indicating a TRPA1-relaxant component.23 A reduced but significant response was observed in the absence of endothelium.23 Xue et al. studied the vasodilatory effect of cinnamaldehyde and its mechanism of action using isolated rings of rat aorta.12 They suggested that cinnamaldehyde dilated both endothelium-intact and endothelium-denuded rings in a dose-dependent manner, which means that cinnamaldehyde-induced vasodilation is related to an endothelium-independent manner.12 Yanaga et al. studied the vasorelaxant effect of cinnamaldehyde using isolated rat aorta and demonstrated that cinnamaldehyde at final concentrations of 1 µM to 1 mM showed dose-dependent relaxation of the rat aorta.24 In the present study, PBF was increased significantly after cinnamaldehyde administration into the feline dental pulp through lingual artery and this response was effectively attenuated by TRPA1 antagonist AP18. This result corresponds to the results of the previous studies and based on these results, cinnamaldehyde-induced vasodilation in dental pulp might be functionally involved with TRPA1 channel activation in dose-dependent manner.
The activation of TRPA1 was proposed to cause arterial dilation through two distinctive pathways.25 First, TRPA1 channels expressed in perivascular nerves are stimulated by chemical agonists and CGRP is released from nerve ending, followed by arterial dilation. Second, TRPA1 channels placed in the myoendothelial junction sites cause endothelium-dependent smooth muscle cell hyperpolarization and vasodilation mediated by Ca2+-activated K+ channels.25 Kunkler et al. studied the role of TRPA1 channels in meningeal vasodilation.26 They suggested that cinnamaldehyde stimulated the release of CGRP from cultured rat trigeminal neurons and this response was blocked by TPRA1 selective antagonist, HC-030031 and CGRP selective antagonist, CGRP8-37.26 This study strongly supported that activation of TRPA1 channels present in primary afferent neurons with cinnamaldehyde led to Ca2+ influx, followed by release of CGRP at nerve endings.27 CGRP binds to G protein-coupled receptors expressed in smooth muscle cells to produce membrane hyperpolarization, myocyte relaxation and vasodilation.28
Cinnamaldehyde is the main component of cinnamon and there are many reports on its pharmacological effects. Mainly, the sedative effect of decreasing spontaneous motor activity,29 anti-inflammatory effects related to cyclooxygenase 2 (COX-2)30 and antibacterial activity against Escherichia coli and Pseudomonas aeruginosa31 have been reported. In addition, in oriental medicine, cinnamaldehyde is often used to improve blood circulation.3233 In relation to dentistry, cinnamaldehyde has been traditionally used as a component of tooth pastes and to manage toothaches, oral microbiota and bad breath.1314
It is interesting to note that cinnamaldehyde has a contradictory character in relation to pain and inflammatory reaction. There have been previous studies about an analgesic effect of cinnamaldehyde. Huang et al. investigated whether cinnamaldehyde prolonged cutaneous analgesia when co-administrated with local anesthetics in rats.34 In this study, cinnamaldehyde alone provided a dose-dependent block to pinpricks and complete block to pinpricks accomplished in 2% cinnamaldehyde with 0.5% lidocaine and with 0.0625% bupivacaine, which were significantly prolonged compared to lidocaine or bupivacaine alone.34 Boonen et al. studied whether cinnamaldehyde inhibited voltage-gated sodium channels expressed in sensory neurons and they found that cinnamaldehyde inhibited tetrodotoxin-sensitive voltage-dependent sodium currents in a concentration dependent manner in mouse trigeminal neurons.35 In a previous study, anti-inflammatory effects of cinnamaldehyde was demonstrated related to COX-2.30 Guo et al. found that cinnamaldehyde reduced interleukin-1β-induced COX-2 activity and consequently inhibited production of prostaglandin E2 in cultured rat cerebral microvascular endothelial cells.30
Markowitz et al. suggested that the biological effects of eugenol, which has similar molecular structure with cinnamaldehyde, varied greatly, depending on its concentration.36 They found that eugenol may have beneficial effects which were prostaglandin synthesis, nerve activity, and white blood cells chemotaxis inhibition with concentrations ranging from 100 nM to 100 µM, but may have cytotoxic effects which were cell death, cell growth, and respiration inhibition with concentration lower than 1 mM.36 In another study, Klein et al. investigated activation of rat trigeminal ganglion cells by sequential application of menthol and/or cinnamaldehyde.37 They found that trigeminal ganglion cells exhibited significant self-desensitization to cinnamaldehyde at 400 µM but not 200 µM and cinnamaldehyde at a concentration of 400 µM but not 200 µM also cross-desensitized menthol-evoked responses.37 The difference of effect of cinnamaldehyde on pain and inflammation might be caused by its concentration and it is needed to clarify the mechanism of action of cinnamaldehyde in pain and inflammatory response.
As mentioned above, cinnamaldehyde has been reported to influence the cardiovascular system. In previous study that evaluated the toxicological effects of cinnamaldehyde, 5 - 10 mg/kg of cinnamaldehyde was applied through vein of Mongrel dogs and decrease of blood pressure and increase of respiratory rate and femoral blood flow were observed.38 In another study, A fall in blood pressure was also observed in male guinea pigs after application of cinnamaldehyde at a dose of 1 mg/kg through intravenous administration.38 Heart rate was reduced by 15% compared to baseline after application of cinnamaldehyde at a dose of 5 mg/kg through vein, while femoral blood flow was observed to be raised.38 In the present study, cinnamaldehyde at a dose of 0.5 mg/kg was administrated into the pulp through lingual artery and it was ascertained that systemic blood pressure was not influenced by this concentration which was much lower than those of previous studies.
Many TRPA1-activating materials including cinnamaldehyde are electrophils which can react with cysteines. The nucleophilic mercapto group of cysteines may attack the α,β-unsaturated bond of cinnamaldehyde and then TRPA1 is activated through covalent binding of cinnamaldehyde to cysteines.39 In the present study, in order to evaluate the role of TRPA1, AP18 was used as a TRPA1 antagonist. While the mechanism of action for AP18 which is an oxime derivative is not exactly confirmed, AP18 may be capable of covalent modification of TRPA1 reversibly, but unable to initiate a conformational change that would lead to channel activation.40 In previous studies, AP18 showed high selectivity and no agonist activity for TRPA1 whereas it was relatively insensitive to other TRP channel family members.41 Petrus et al. showed dose-response relationships for block of the calcium influx by AP18 into CHO cells expressing mouse and human TRPA1 elicited by cinnamaldehyde.42 Moreover, AP18 reversibly blocked cinnamaldehyde-induced TRPA1 currents in excised patches from Xenopus oocytes.42 Importantly, AP18 significantly blocked cinnamaldehyde-induced but not capsaicin-induced nociceptive events which are related to TRPV1, demonstrating efficacy and specificity.42 In the previous study in our lab, AP18 was applied to dental pulp through the dentinal cavity, not through the lingual artery and it resulted in significant inhibition of TRPA1 activation. Based on these studies, AP18 was determined to be administered through dentinal tubule in the present study.
The results of the present study provided a functional evidence that TRPA1 is involved in the mechanism of cinnamaldehyde-induced vasodilation in the feline dental pulp. Further study is needed to clarify the mediation of CGRP in the cinnamaldehyde-induced vasodilation in the dental pulp.
Figures and Tables
Figure 1
Schematic diagram of experiment design. Pulpal blood flow was measured at tooth surface by a laser Doppler flowmeter. Femoral artery was cannulated for monitoring of systemic blood pressure and femoral vein was cannulated for injection of supplemental anesthetics. TRPA1 antagonist was administered through the dentinal cavity and cinnamaldehyde was injected into the pulp through the lingual artery. TRPA1, transient receptor potential ankyrin 1.
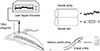
Figure 2
Typical strip-chart recordings of systemic blood pressure and pulpal blood flow (PBF). (a) Cinnamaldehyde (0.5 mg/kg, i.a.) resulted in a significant increase of PBF; (b) TRPA1 antagonist, AP18 (2.5 - 3.0 mM, i.c.), effectively attenuated an increase in PBF induced by cinnamaldehyde (0.5 mg/kg, i.a.) (p < 0.05). i.a., intra-arterial; i.c., into the dentinal cavity.

Table 1
Effect of cinnamaldehyde on PBF

| No. | % Change of PBF (mean ± SEM) | |
|---|---|---|
| Ethanol (70%) + DMSO (30%) (cinnamaldehyde vehicle, i.a.) | 19 | 7.63 ± 1.17a |
| Cinnamaldehyde (0.5 mg/kg, i.a.) | 19 | 88.02 ± 11.56b |
Acknowledgement
This research was supported by Kyungpook National University Research Fund 2012 (2013, 2014).
References
1. Hargreaves KM, Cohen S, Berman LH. Cohen's pathways of pulp. 10th ed. St. Louis: Mosby Elsevier;2011. p. 571.
2. Heyeraas KJ, Kim S, Raab WH, Byers MR, Liu M. Effect of electrical tooth stimulation on blood flow, interstitial fluid pressure and substance P and CGRP-immunoreactive nerve fibers in the low compliant cat dental pulp. Microvasc Res. 1994; 47:329–343.

3. Nyman E, Franzén B, Nolting A, Klement G, Liu G, Nilsson M, Rosén A, Björk C, Weigelt D, Wollberg P, Karila P, Raboisson P. In vitro pharmacological characterization of a novel TRPA1 antagonist and proof of mechanism in a human dental pulp model. J Pain Res. 2013; 6:59–70.
4. Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with delta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005; 493:596–606.

5. Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009; 29:1568–1578.

6. El Karim IA, Linden GJ, Curtis TM, About I, McGahon MK, Irwin CR, Killough SA, Lundy FT. Human dental pulp fibroblasts express the 'cold-sensing' transient receptor potential channels TRPA1 and TRPM8. J Endod. 2011; 37:473–478.

7. Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch. 2012; 464:425–458.

8. Radresa O, Dahllof H, Nyman E, Nolting A, Albert JS, Raboisson P. Roles of TRPA1 in pain pathophysiology and implications for the development of a new class of analgesic drugs. Open Pain J. 2013; 6:137–153.

9. Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003; 112:819–829.

10. Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004; 427:260–265.

11. Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004; 41:849–857.

12. Xue YL, Shi HX, Murad F, Bian K. Vasodilatory effects of cinnamaldehyde and its mechanism of action in the rat aorta. Vasc Health Risk Manag. 2011; 7:273–280.
13. Aneja KR, Joshi R, Sharma C. Antimicrobial activity of Dalchini (Cinnamomum zeylanicum bark) extracts on some dental caries pathogens. J Pharm Res. 2009; 2:1387–1390.
14. Gupta C, Kumari A, Garg AP, Catanzaro R, Marotta F. Comparative study of cinnamon oil and clove oil on some oral microbiota. Acta Biomed. 2011; 82:197–199.
15. Silva CR, Oliveira SM, Rossato MF, Dalmolin GD, Guerra GP, da Silveira Prudente A, Cabrini DA, Otuki MF, André E, Ferreira J. The involvement of TRPA1 channel activation in the inflammatory response evoked by topical application of cinnamaldehyde to mice. Life Sci. 2011; 88:1077–1087.

16. Liu MT, Bioltto G, Markowitz K, Kim S. Changes in coronal pulpal blood flow following a class V cavity preparation. J Dent Res. 1987; 66:Supplment 1. Abstract #558. 76.
17. Kim SK, Ang L, Hsu YY, Dörcher-Kim J, Kim S. Antagonistic effect of D-myo-inositol-1,2,6-trisphosphate (PP56) on neuropeptide Y-induced vasoconstriction in the feline dental pulp. Arch Oral Biol. 1996; 41:791–798.

18. Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008; 586:3447–3459.

19. Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008; 118:1899–1910.

20. Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009; 106:1273–1278.
21. Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007; 27:11412–11415.

22. Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andrè E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007; 104:13519–13524.

23. Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain SD. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res. 2010; 87:760–768.

24. Yanaga A, Goto H, Nakagawa T, Hikiami H, Shibahara N, Shimada Y. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol Pharm Bull. 2006; 29:2415–2418.

26. Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011; 152:38–44.

27. Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006; 124:1269–1282.

28. Högestätt ED, Johansson R, Andersson DA, Zygmunt PM. Involvement of sensory nerves in vasodilator responses to acetylcholine and potassium ions in rat hepatic artery. Br J Pharmacol. 2000; 130:27–32.

29. Harada M, Ozaki Y. Pharmacological studies on Chinese cinnamon. I. Central effects of cinnamaldehyde. Yakugaku Zasshi. 1972; 92:135–140.

30. Guo JY, Huo HR, Zhao BS, Liu HB, Li LF, Ma YY, Guo SY, Jiang TL. Cinnamaldehyde reduces IL-1beta-induced cyclooxygenase-2 activity in rat cerebral microvascular endothelial cells. Eur J Pharmacol. 2006; 537:174–180.

31. Chang ST, Chen PF, Chang SC. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J Ethnopharmacol. 2001; 77:123–127.

32. Takenaga M, Hirai A, Terano T, Tamura Y, Kitagawa H, Yoshida S. In vitro effect of cinnamic aldehyde, a main component of Cinnamomi Cortex, on human platelet aggregation and arachidonic acid metabolism. J Pharmacobiodyn. 1987; 10:201–208.

33. Huang J, Wang S, Luo X, Xie Y, Shi X. Cinnamaldehyde reduction of platelet aggregation and thrombosis in rodents. Thromb Res. 2007; 119:337–342.

34. Huang CC, Won HT, Cheng JK, Chen CC, Hung YC. Cinnamaldehyde prolongs cutaneous analgesia of local anesthetics in rats. Anesthesiology 2011. In : American Society of Anesthesiologists Annual Meeting (Regional anesthesia and acute pain; p. Abstract #A991. updated 2016 Jul 29. Available from: http://www.asaabstracts.com/strands/asaabstracts/abstractList.htm;jsessionid=43A31BC5F56A2DDB2462F253453561B4?year=2011&index=17.
35. Boonen B, Alpizar YA, Benoy V, Van den Bosch L, Voets T, Talavera K. The Trpa1 agonist cinnamaldehyde acts as a local anesthetic inhibiting voltage-gated sodium channels in sensory neurons. Biophys J. 2014; 106:326a–327a.

36. Markowitz K, Moynihan M, Liu M, Kim S. Biologic properties of eugenol and zinc oxide-eugenol. A clinically oriented review. Oral Surg Oral Med Oral Pathol. 1992; 73:729–737.
37. Klein AH, Carstens MI, Zanotto KL, Sawyer CM, Ivanov M, Cheung S, Carstens E. Self- and cross-desensitization of oral irritation by menthol and cinnamaldehyde (CA) via peripheral interactions at trigeminal sensory neurons. Chem Senses. 2011; 36:199–208.

38. Cocchiara J, Letizia CS, Lalko J, Lapczynski A, Api AM. Fragrance material review on cinnamaldehyde. Food Chem Toxicol. 2005; 43:867–923.

39. Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007; 445:541–545.

40. Defalco J, Steiger D, Gustafson A, Emerling DE, Kelly MG, Duncton MA. Oxime derivatives related to AP18: agonists and antagonists of the TRPA1 receptor. Bioorg Med Chem Lett. 2010; 20:276–279.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download