Abstract
Objectives
A variety of root canal sealers were recently launched to the market. This study evaluated physicochemical properties, biocompatibility, and sealing ability of a newly launched resin-based sealer (Dia-Proseal, Diadent) compared to the existing root canal sealers (AHplus, Dentsply DeTrey and ADseal, Metabiomed).
Materials and Methods
The physicochemical properties of the tested sealers including pH, solubility, dimensional change, and radiopacity were evaluated. Biocompatibility was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For microleakage test, single-rooted teeth were instrumented, and obturated with gutta-percha and one of the sealers (n = 10). After immersion in 1% methylene blue solution for 2 weeks, the specimens were split longitudinally. Then, the maximum length of staining was measured. Statistical analysis was performed by one-way analysis of variance followed by Tukey test (p = 0.05).
Results
Dia-Proseal showed the highest pH value among the tested sealers (p < 0.05). ADseal showed higher dimensional change compared to AHplus and Dia-Proseal (p < 0.05). The solubility values of AHplus and Dia-Proseal were similar, whereas ADseal had the lowest solubility value (p < 0.05). The flow values of sealer in increasing order were AHplus, DiaProseal, and ADseal (p < 0.05). The radiopacity of AHplus was higher than those of ADseal and Dia-Proseal (p < 0.05). The cell viability of the tested materials was statistically similar throughout the experimental period. There were no significant differences in microleakage values among the tested samples.
Three dimensional filling of the root canal is essential for preventing reinfection of the root canal. Ideal endodontic sealer helps preventing leakage, reducing the possibility of reinfection, and healing of the periapical lesion.1 According to Grossman,2 an ideal root canal sealer should possess excellent sealing ability, dimensional stability, insolubility, and biocompatibility. A great variety of endodontic sealers are available commercially with materials such as zinc oxide eugenol, epoxy resin, glass ionomer, and calcium hydroxide. Among these sealers, resin-based sealers possess acceptable physical and biological properties.3 AH series is one of the successful resin-based sealers that was developed more than 50 years ago.4 The improved AHplus (Dentsply DeTrey, Konstanz, Germany) is a 2 component paste/paste sealer that has been used frequently in an experiment as a well-established sealer with excellent physicochemical properties.567
Recently, a new root canal sealer has been introduced to substitute conventional sealers with the guarantee of improved clinical performance (Dia-Proseal, Diadent, Cheongju, Korea). When a new endodontic sealer is launched, clinicians may seek for the information regarding its physicochemical properties, biocompatibility, and root canal sealing ability. However, little information about Dia-Proseal is available to the dentists. Therefore, this study was aimed to evaluate the physical properties, biocompatibility, and root canal sealing ability of this new root canal sealer, and to compare with AHplus and another root canal sealer (ADseal, Metabiomed, Cheongju, Korea). Our null hypothesis was as follows: There is no difference between these three tested root canal sealers with respect to physicochemical properties, biocompatibility, and root canal sealing ability.
The three root canal sealers included in this study were AHplus, ADseal, and Dia-Proseal. The chemical composition of the tested materials is shown in Table 1.
The pH was measured according to the criteria used in a previously published study.8 The sealer samples with 1 mm thickness and 5 mm diameter were prepared and allowed to set for 1 day (n = 3). After setting, each specimen was immersed in glass tubes containing 10 mL deionized water. Then, the pH was measured with a pH meter (Orion 3 star, Thermo Scientific, Singapore) previously calibrated with pH 7.0 and 4.0 solutions after 1, 2, 3, 7, 10, and 14 days.
The dimensional change was measured using the method recommended by ISO 6876/2012. Each sealer was put into a cylindrical silicon mold with a diameter of 6 mm and a height of 12 mm (n = 5). After setting, the mold was measured for length (M1) with a micrometer caliper (accuracy of 10 µm). The samples were then stored in distilled water at 37℃. After 7, 14, and 21 days, the length (M2) was measured. The changes in length were measured 3 times, and mean values were recorded as the dimensional change using the following formula: Dimensional change = (M2 - M1) / M1 × 100.
The solubility was measured using the method recommended by ISO 6876/2012. Paraffin wax mold in 1.5 mm thickness and inner diameter of 20 mm was used for each sealer (n = 5). The molds were filled with the sealers and the assembly was placed in an incubator (37℃, > 95% relative humidity) for a period of time 100% longer than the setting time. After the sealers were removed from the mold, they were weighed 3 times each in analytical balance (HM-200, A&D Engineering Inc., Bradford, MA, USA). The mean weight was recorded as W1. Then, the specimens were immersed in tubes containing 10 mL of distilled water for 7 days. After this period, the specimens were dried with absorbent paper and placed in a dessicator, and its weight was recorded as W2. The solubility of the sealer was calculated using following formula: Solubility = (W1 - W2) / W1 × 100.
The flow was assessed using the method recommended by ISO 6876/2012. A volume of 0.5 mL sealer was put on a glass plate (n = 3). After 180 ± 5 seconds, the second glass plate was placed centrally on top of the sealer to make a total mass on the plate of 120 g. Ten minutes after mixing the sealer, the load was removed and the average of the major and minor diameters of the compressed sealer was measured by a digital caliper. The mean of 3 measurements for each sample was taken as the flow of the sealer.
The radiopacity was evaluated using the method recommended by ISO 6876/2012. Cylindrical samples from each material were fabricated by pouring the manipulated sealers into metallic rings with 10 mm internal diameter and 1 mm thickness (n = 3). The filled rings were kept at 37℃ until cements were set completely. The specimens were placed on occlusal x-ray film (Kodak Insight, Rochester, NY, USA) with an aluminum step-wedge graduated from 1 to 10 mm (in 1 mm increments). A Kodak-2200 X-ray machine (Kodak) operating at 70 kV, 10 mA, 18 pulses/second and with a focus-sensor distance of 30 cm was used. The radiographs were digitized and analyzed using a densitometer (GS-800, Bio-Rad, Hercules, CA, USA).
The tested material was placed into a silicon mold (1 mm thickness and 10 mm diameter). After setting, the cement was removed from the mold and stored in 10 mL of minimal essential medium-α (MEM-α, HyClone Laboratories, Logan, UT, USA) containing 10% fetal bovine serum (FBS, HyClone Laboratories) for 3 days.
L929 cell line was purchased from Korean Cell Line Bank, Seoul, Korea. The cells were seeded in 24-well plates (2 × 104 cells/well) and pre-incubated in growth medium for 24 hours (n = 4). After overnight attachment, cells were treated with the prepared extracts of sealers for 1, 3, 7, and 14 days. Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Briefly, 200 µL of MTT solution (0.5 mg/mL in phosphate buffered saline) (Amresco, Solon, OH, USA) was added to each well, and the wells were incubated for 2 hours. Subsequently, 200 µL of dimethyl sulfoxide (Amresco) was added to each well. Reduced MTT was then measured spectrophotometrically at 540 nm in a dual-beam microtiter plate reader (SPECTROstar Nano, BMG Labtech, Ortenberg, Germany).
Thirty-four freshly extracted mandibular premolars with straight and single root canal were used. After access openings, a size 15 file was inserted into the root canal. Working length was determined by placing a size 15 K-file into the canal until it became visible at the apical foramen and then decreasing the file length by 1 mm. The root canals were shaped with ProTaper rotary files up to an F3. During the preparation, the root canal was irrigated with 5 mL 3.0% sodium hypochlorite (NaOCl). After instrumentation, 17% ethylenediaminetetraacetic acid (EDTA) was applied for 1 minute to remove the smear layer. The root canals were dried with paper points. Samples were randomly allocated into three groups (Dia-Proseal, AHplus, and ADseal, n = 10) while setting positive (n = 2) and negative (n = 2) controls. All teeth were obturated to its working length by vertical compaction of 0.06 taper guttapercha cones and sealers. The access cavity was sealed with composite resin, and the samples were stored for 24 hours in 100% relative humidity at 37℃. The root surfaces were coated with two layers of nail polish except for the apical 3 mm. Then, the samples were stored in 1% methylene blue dye at 37℃ for 2 weeks. After removal from the dye, the samples were washed with distilled water. The root of each tooth was sectioned longitudinally using a diamond disc, and each half was analyzed under a stereomicroscope (Leica MZ16FA, Leica, Wetzlar, Germany). The amount of leakage was measured from the working length to the most coronal part of the root canal to which the dye had penetrated. Two independent measurements were made for each tooth. The microleakage experimental procedures were approved by the Institutional Review Board of the Chonbuk National University Hospital (IRB No.: 2014-10-001).
Dia-Proseal showed significantly higher pH value compared to other sealers (p < 0.05, Figure 1a). ADseal showed significantly higher dimensional change compared to AHplus and Dia-Proseal (p < 0.05, Figure 1b). The solubility values of AHplus and Dia-Proseal were similar, whereas ADseal showed the lowest solubility value (p < 0.05, Figure 1c). The flow values of sealer in increasing order were AHplus, Dia-Proseal, and ADseal, and there was statistical significance (p < 0.05, Figure 1d). The radiopacity of AHplus was higher than ADseal and Dia-Proseal (p < 0.05, Figure 2).
The cell viability of the tested materials were similar throughout the experimental period (Figure 3).
There was no significant difference in microleakage value among the tested samples (Figure 4).
New root canal sealers have been continuously introduced into the endodontic market. Dia-Proseal is one of those new root canal sealers. According to the manufacturer, it has several characteristics such as fast-setting time, volume stability, good sealing of complex root canal system, long-term storage ability, and dual syringe system allowing easy mixture. Nevertheless, it should be compared with preexisting sealers because clinicians need fair results compared to the data provided by the manufacturer. Here, several standardized tests were performed to evaluate the properties of Dia-Proseal.
In this study, Dia-Proseal showed the highest pH value among three different root canal sealers (Figure 1a). According to the manufacturer, Dia-Proseal contains calcium hydroxide which may influence on the higher pH value (Table 1). The high pH value of root canal sealer is important due to its relation to disinfection of root canal. Furthermore, the high level of pH can neutralize the acids secreted by osteoclasts and it can also destruct bacterial membrane and its protein structure.9 Therefore, Dia-Proseal can be considered to possess better antimicrobial activity than other tested sealers.
Dimensional change demonstrates the shrinkage or expansion of the material after setting in percentage. In this study, all the tested sealers showed slight increase in their volume (Figure 1b). ADseal showed the highest dimensional change among the subjects, while Dia-Proseal showed the lowest change. The result can be explained by water absorption after polymerization. Slight expansion of root canal sealer may contribute to improving sealing ability, but excessive one is unfavorable since it may cause cracks in the root after its application.10
In solubility test, the results of all the tested materials satisfied the criteria set by ISO that solubility of root canal sealer should not exceed 3% by mass (Figure 1c). ADseal showed an odd result of having minus percentage in solubility. Such result can be interpreted that ADseal is highly hygroscopic unlike AHplus or Dia-Proseal, and may be closely related to significantly higher dimensional change of ADseal. However, Marciano et al. reported that ADseal had no significant difference in solubility with AHplus. This discrepancy might be caused by inaccuracy of the traditional methods for measurement of solubility and dimensional change.11 Recently, there is a novel approach to evaluate solubility and dimensional change more precisely by using micro-computed tomographic (micro-CT) scanning.12 In this respect, it is required to measure the physical properties with more advanced methods.
An adequate flow is an important characteristic of root canal sealer to seal apical foramen and spaces between gutta-percha cone and dentinal wall. However, an excessive flow increases the risk of sealer extrusion into periodontal tissue.13 Our result on the flow of each endodontic sealer showed that ADseal had significantly higher flowability than either AHplus or Dia-Proseal (Figure 1d). However, Marciano et al. investigated physical properties including flowability of three epoxy resin-based sealers (AHplus, ADseal, and Acroseal), and their results showed no significant difference between AHplus and ADseal.11 Indeed, most previous studies including our investigation on the flow of root canal sealers used the simple press method. Chang et al. reported that the viscosity measured using a rheometer was more precise than the flowabilities measured by the simple press method.14 Therefore, a rheological study is required to evaluate the flowabilities of these sealers.
A root canal sealer should be radiopaque to enable visualization and assessment on the radiograph. According to ISO standard, a minimal radiopacity of root canal sealer has to be equivalent to 3 mm of aluminum. In this study, all the tested materials provided sufficient radiopacity satisfying the standard of ISO (Figure 2).
Root canal sealers are often placed in close contact with periapical tissues. Hence, favorable biocompatibility is desirable because it can heal proximal periapical tissue or activate new bone formation. As shown in Figure 3, there were no differences statistically on cell viability of AHplus, ADseal, and Dia-Proseal. Furthermore, this result showed cell viability of more than 80%. Therefore, Dia-Proseal has acceptable biocompatibility as conventional root canal sealer.
Leakage is one of the major reasons for the failure of endodontic treatment.15 It is inevitable that root canal sealer leaks to some extent. Most leakage occurs between dentinal walls and the sealer. Therefore, the ability of the sealer to bond to dentinal walls is important to minimize the leakage. Different evaluations using dyes,1617 bacteria,1819 fluid filtration,2021 and glucose penetration22 have been performed to assess the leakage. Dye penetration test have frequently been used for leakage assessment. However, in some reports, correlation between clinical performance and apical dye penetration has been questioned.2324 Nonetheless, other previous literatures showed a good interrelation between dye penetration and other leakage tests.2526 As shown in Figure 4, there were no statistically significant differences among the sealers on microleakage. All the tested materials showed similar sealing ability although ADseal showed more microleakage than the others.
The results of this study showed that DiaProseal showed suitable physicochemical properties, cell viability, and root canal sealing ability. However, further evaluation with more precise and advanced methods is required regarding the measurement of the physical properties and root canal sealing ability.
Figures and Tables
 | Figure 1Changes in physicochemical properties of the tested sealers.(a) pH (n = 3); (b) dimensional change (n = 5); (c) solubility (n = 5); (d) flow (n = 3). Groups identified by the same symbols were not significantly different in the same gene group. AH, AHplus; AD, ADseal; DP, Dia-Proseal.
|
 | Figure 2Radiopacity of the tested materials (n = 3).(a) Radiograph showing the radiopacity of each material and its equivalence to that of the aluminum step-wedge; (b) Relative radiographic density of each material in comparison with that of a 10-step aluminum step-wedge. Groups identified by the same symbols were not significantly different in the same gene group. AH, AHplus; AD, ADseal; DP, Dia-Proseal.
|
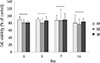 | Figure 3Biocompatibility of the tested materials (n = 4).Cell viability tested by the MTT assay. Groups connected with the bar were not significantly different in the same gene group. AH, AHplus; AD, ADseal; DP, Dia-Proseal.
|
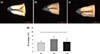 | Figure 4Representative photographs showing the measurement of the apical dye penetration of (a) AHplus, (b) ADseal, and (c) DiaProseal; (d) Average length of dye penetration.Values are shown as the mean ± standard deviation (n = 10). There was no significant difference in microleakage value among the tested groups. AH, AHplus; AD, ADseal; DP, Dia-Proseal.
|
Table 1
Chemical composition of the endodontic sealers used in this study

Acknowledgement
This paper was supported by the Biomedical Research Institute of the Chonbuk National University Hospital in 2015.
References
1. Lucena-Martín C, Ferrer-Luque CM, González-Rodríguez MP, Robles-Gijón V, Navajas-Rodríguez de. A comparative study of apical leakage of Endomethasone, Topseal and Roeko Seal cements. J Endod. 2002; 28:423–426.
2. Grossman LI. Endodontic practice. 10th ed. Philadelphia: Henry Kimpton Publishers;1981. p. 297.
3. Donnelly A, Sword J, Nishitani Y, Yoshiyama M, Agee K, Tay FR, Pashley DH. Water sorption and solubility of methacrylate resin-based root canal sealers. J Endod. 2007; 33:990–994.

4. Schröeder A. The impermeability of root canal filling material and first demonstrations of new root filling materials. SSO Schweiz Monatsschr Zahnheilkd. 1954; 64:921–931.
5. Versiani MA, Carvalho-Junior JR, Padilha MI, Lacey S, Pascon EA, Sousa-Neto MD. A comparative study of physicochemical properties of AH Plus and Epiphany root canal sealants. Int Endod J. 2006; 39:464–471.

6. Bouillaguet S, Shaw L, Barthelemy J, Krejci I, Wataha JC. Long-term sealing ability of Pulp Canal Sealer, AH Plus, GuttaFlow and Epiphany. Int Endod J. 2008; 41:219–226.

7. Ørstavik D. Materials used for root canal obturation: technical, biological and clinical testing. Endod Topics. 2005; 12:25–38.

8. Lim ES, Park YB, Kwon YS, Shon WJ, Lee KW, Min KS. Physical properties and biocompatibility of an injectable calcium-silicate-based root canal sealer: in vitro and in vivo study. BMC Oral Health. 2015; 15:129.

9. Silva EJ, Rosa TP, Herrera DR, Jacinto RC, Gomes BP, Zaia AA. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod. 2013; 39:274–277.

10. Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and portland cement. J Endod. 2006; 32:193–197.

11. Marciano MA, Guimarães BM, Ordinola-Zapata R, Bramante CM, Cavenago BC, Garcia RB, Bernardineli N, Andrade FB, Moraes IG, Duarte MA. Physical properties and interfacial adaptation of three epoxy resin-based sealers. J Endod. 2011; 37:1417–1421.

12. Silva EJ, Perez R, Valentim RM, Belladonna FG, De-Deus GA, Lima IC, Neves AA. Dissolution, dislocation and dimensional changes of endodontic sealers after a solubility challenge: a micro-CT approach. Int Endod J. 2016; 03. 22. DOI: 10.1111/iej.12636. [Epub ahead of print].

13. Almeida JF, Gomes BP, Ferraz CC, Souza-Filho FJ, Zaia AA. Filling of artificial lateral canals and microleakage and flow of five endodontic sealers. Int Endod J. 2007; 40:692–699.

14. Chang SW, Lee YK, Zhu Q, Shon WJ, Lee WC, Kum KY, Baek SH, Lee IB, Lim BS, Bae KS. Comparison of the rheological properties of four root canal sealers. Int J Oral Sci. 2015; 23:56–61.

15. Kim YK, Grandini S, Ames JM, Gu LS, Kim SK, Pashley DH, Gutmann JL, Tay FR. Critical review on methacrylate resin-based root canal sealers. J Endod. 2010; 36:383–399.

16. Zmener O, Pameijer CH, Macri E. Evaluation of the apical seal in root canals prepared with a new rotary system and obturated with a methacrylate based endodontic sealer: an in vitro study. J Endod. 2005; 31:392–395.

17. Aptekar A, Ginnan K. Comparative analysis of microleakage and seal for 2 obturation materials: Resilon/Epiphany and gutta-percha. J Can Dent Assoc. 2006; 72:245.
18. De-Deus G, Audi C, Murad C, Fidel S, Fidel RA. Sealing ability of oval-shaped canals filled using the System B heat source with either gutta-percha or Resilon: an ex vivo study using a polymicrobial leakage model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:e114–e119.
19. Baumgartner G, Zehnder M, Paqué F. Enterococcus faecalis type strain leakage through root canals filled with Gutta-Percha/AH plus or Resilon/Epiphany. J Endod. 2007; 33:45–47.

20. Tunga U, Bodrumlu E. Assessment of the sealing ability of a new root canal obturation material. J Endod. 2006; 32:876–878.

21. Sagsen B, Er O, Kahraman Y, Orucoglu H. Evaluation of microleakage of roots filled with different techniques with a computerized fluid filtration technique. J Endod. 2006; 32:1168–1170.

22. Kaya BU, Kececi AD, Belli S. Evaluation of the sealing ability of gutta-percha and thermoplastic synthetic polymer-based systems along the root canals through the glucose penetration model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:e66–e73.

23. Wu MK, Wesselink PR. Endodontic leakage studies reconsidered. Part I. Methodology, application and relevance. Int Endod J. 1993; 26:37–43.

24. Oliver CM, Abbott PV. Correlation between clinical success and apical dye penetration. Int Endod J. 2001; 34:637–644.

25. Delivanis PD, Chapman KA. Comparison and reliability of techniques for measuring leakage and marginal penetration. Oral Surg Oral Med Oral Pathol. 1982; 53:410–416.

26. Martell B, Chandler NP. Electrical and dye leakage comparison of three root-end restorative materials. Quintessence Int. 2002; 33:30–34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download