Abstract
Objectives
Materials and Methods
Results
Figures and Tables
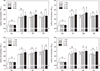 | Figure 1Mean Vickers microhardness of specimen surface after fluoride applications (n = 4). Bars indicate standard deviations. Non, Untreated; FP, Fluor Protector; FPN, Fluor Protector + 2% sodium fluoride (NaF); FPS, Fluor Protector + 8% tin(ii) fluoride (SnF2); TM, Tooth Mousse Plus; TMN, Tooth Mousse Plus + 2% NaF; TMS, Tooth Mousse Plus + 8% SnF2; A, 60 Second Gel; AN, 60 Second Gel + 2% NaF; AS, 60 Second Gel + 8% SnF2; CS, CavityShield; CSN, CavityShield + 2% NaF; CSS, CavityShield + 8% SnF2.a - d, Groups with different letters were significantly different (p < 0.05).
HV0.2, Hardness values were determined using a digital microhardness tester with a load of 0.2 kg.
|
 | Figure 2Fluoride release after fluoride applications (n = 5). Bars indicate standard deviations. Non, Untreated; FP, Fluor Protector; FPN, Fluor Protector + 2% sodium fluoride (NaF); FPS, Fluor Protector + 8% tin(ii) fluoride (SnF2); TM, Tooth Mousse Plus; TMN, Tooth Mousse Plus + 2% NaF; TMS, Tooth Mousse Plus + 8% SnF2; A, 60 Second Gel; AN, 60 Second Gel + 2% NaF; AS, 60 Second Gel + 8% SnF2; CS, CavityShield; CSN, CavityShield + 2% NaF; CSS, CavityShield + 8% SnF2. |
 | Figure 3Calcium content of specimens after fluoride applications by EPMA analysis. Bars indicate standard deviations. Non, Untreated; FP, Fluor Protector; FPN, Fluor Protector + 2% sodium fluoride (NaF); FPS, Fluor Protector + 8% tin(ii) fluoride (SnF2); TM, Tooth Mousse Plus; TMN, Tooth Mousse Plus + 2% NaF; TMS, Tooth Mousse Plus + 8% SnF2; A, 60 Second Gel; AN, 60 Second Gel + 2% NaF; AS, 60 Second Gel + 8% SnF2; CS, CavityShield; CSN, CavityShield + 2% NaF; CSS, CavityShield + 8% SnF2.a - d, Groups with different letters were significantly different (p < 0.05).
|
 | Figure 4Phosphorus content of specimens after fluoride applications by EPMA analysis. Bars indicate standard deviations. Non, Untreated; FP, Fluor Protector; FPN, Fluor Protector + 2% sodium fluoride (NaF); FPS, Fluor Protector + 8% tin(ii) fluoride (SnF2); TM, Tooth Mousse Plus; TMN, Tooth Mousse Plus + 2% NaF; TMS, Tooth Mousse Plus + 8% SnF2; A, 60 Second Gel; AN, 60 Second Gel + 2% NaF; AS, 60 Second Gel + 8% SnF2; CS, CavityShield; CSN, CavityShield + 2% NaF; CSS, CavityShield + 8% SnF2.a - c, Groups with different letters were significantly different (p < 0.05).
|
Table 1
Fluoride products used in this study

Table 2
Fluoride application methods on specimens followed in this study (n = 10)





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download