Abstract
Objectives
Sodium hypochlorite (NaOCl) is an excellent bactericidal agent, but it is detrimental to stem cell survival, whereas intracanal medicaments such as calcium hydroxide (Ca[OH]2) promote the survival and proliferation of stem cells. This study evaluated the effect of sequential NaOCl and Ca[OH]2 application on the attachment and differentiation of dental pulp stem cells (DPSCs).
Materials and Methods
DPSCs were obtained from human third molars. All dentin specimens were treated with 5.25% NaOCl for 30 min. DPSCs were seeded on the dentin specimens and processed with additional 1 mg/mL Ca[OH]2, 17% ethylenediaminetetraacetic acid (EDTA) treatment, file instrumentation, or a combination of these methods. After 7 day of culture, we examined DPSC morphology using scanning electron microscopy and determined the cell survival rate with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. We measured cell adhesion gene expression levels after 4 day of culture and odontogenic differentiation gene expression levels after 4 wk using quantitative real-time polymerase chain reaction.
Results
DPSCs did not attach to the dentin in the NaOCl-treated group. The gene expression levels of fibronectin-1 and secreted phosphoprotein-1 gene in both the Ca[OH]2- and the EDTA-treated groups were significantly higher than those in the other groups. All Ca[OH]2-treated groups showed higher expression levels of dentin matrix protein-1 than that of the control. The dentin sialophosphoprotein level was significantly higher in the groups treated with both Ca[OH]2 and EDTA.
Revascularization has recently been proposed as an alternative treatment option for immature permanent teeth with necrotic pulp. Many clinical case reports have shown that revascularization prevents clinical symptoms, heals periapical lesions, and increases dentinal wall thickness and root length as shown on radiographs.123 The ideal and final objective of regenerative endodontic treatment is complete dental pulp regeneration. In other words, new vital pulp tissue can regenerate in empty but infected root canal spaces, reaching the coronal pulp chamber. Therefore, pulp regeneration is considered an ideal treatment to maintain tooth homeostasis, prevent reinfection and fractures, and preserve tooth longevity.
Disinfection of the entire infected root canal is essential for successful regenerative endodontic treatment. This disinfection requires the selection of proper irrigants based on both antimicrobial properties and the proliferative capacity of the stem cells. Sodium hypochlorite (NaOCl) is the most commonly used irrigant in regenerative endodontic treatments.1456 It is an excellent bactericidal agent. However, it is cytotoxic to human periodontal ligament stem cells, cultured fibroblasts, stem cells from human exfoliated deciduous teeth, stem cells from apical papilla, and dental pulp stem cells (DPSCs).7891011 Both in vitro and in vivo studies have shown that NaOCl has a significant negative effect on stem cell survival, attachment, and differentiation.7891011
Previous studies have examined methods for neutralizing NaOCl cytotoxicity using various irrigation protocols with different concentrations of NaOCl and neutralizing agents.6711 Commonly used intracanal medicaments in regenerative endodontic treatments are triple antibiotic paste, double antibiotic paste, calcium hydroxide (Ca[OH]2), formocresol, and amoxicillin/clavulanic acid (Augmentin, Champs Pharmacy, San Antonio, TX, USA).121314 These medicaments can also affect the survival and differentiation of the DPSCs. Recent studies have investigated the effects of the intracanal medicaments such as triple antibiotic paste and double antibiotic paste on proliferation.1516
Currently, no known method exists to decrease the cytotoxicity of residual NaOCl. Moreover, the effect of sequential application of NaOCl and Ca(OH)2 on DPSC survival has not been investigated. Therefore, the purpose of this study was to evaluate the effect of the sequential use of NaOCl and Ca(OH)2 on the attachment and differentiation of DPSCs. Furthermore, we investigated the optimal protocols for reducing the cytotoxicity of NaOCl on DPSCs.
The protocols of our experiment were taken directly from an earlier study.17 The present study was approved by the Institutional Review Board of Yonsei Dental Hospital, Seoul, Republic of Korea (IRB 2-2012-0055). Normal human third molars were collected from young adults (aged 16 - 22 years) treated in the Department of Advanced General Dentistry, Yonsei University Dental Hospital. The pulp tissue was separated from the apex of the extracted third molars with a barbed broach and cut into 1 mm3 blocks and placed in 60 mm culture dishes (BD Falcon, Franklin Lakes, NJ, USA) with a counting chamber cover glass (Marienfeld-Superior, Lauda-Königshofen, Germany) to allow for the outgrowth of cells. The tissues were cultured in α-modified Eagle medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen), 2 mM/L glutamine, 100 µM/L ascorbic acid-2-phosphate (WAKO, Tokyo, Japan), 100 U/mL penicillin, and 100 µg/mL streptomycin (Biofluids, Rockville, MD, USA) at 37℃ in 5% CO2 incubator. The outgrowth cells were transferred to 5 × 10 cm culture flasks (passage 1) and grown to confluence. The cells were then harvested and kept frozen in liquid N2 tank. Cells from passage 3 were used in this experiment.
The expression of mesenchymal stem cell surface antigen was analyzed by flow cytometry. The dental pulp cells (1 × 106) were harvested in phosphate-buffered saline (PBS, Mediatech Inc., Manassas, VA, USA), washed, and resuspended in the flow cytometry staining buffer (eBiosciences, San Diego, CA, USA). They were incubated with fluorescein isothiocyanate (FITC)-labeled mouse antihuman antibodies (CD146-FITC, CD90-FITC, CD105-PE, CD34-FITC, and CD45-PE, all were supplied by eBiosciences) or antihuman Stro-1 (DyLight 488, BD Biosciences, San Jose, CA, USA) for 1 hour at 4℃. The expression profiles were examined using an LSR II flow cytometer system (BD Biosciences). At least 50,000 cells were used for fluorescence-activated cell sorting analysis. We chose the appropriate cell lines according to the minimal criteria for defining multipotent mesenchymal stem cells, which are hereafter referred to as DPSCs.
Dentin slices were prepared from the normal human third molars. The coronal dentin was cut into a disc shape approximately 1 mm thick with a low-speed diamond cutter (RB205 Metsaw-LS, R&B Inc., Daejeon, Korea) under sterile phosphate-buffered saline (PBS) irrigation (Mediatech Inc.). The slices were processed with ethylene oxide gas sterilization and ethylenediaminetetraacetic acid (EDTA) treatment to remove the smear layer produced during specimen preparation. All dentin slices were washed 5 times with PBS and placed in sterile 100 × 15 mm Nunclon cell culture dishes (NUNC, Roskilde, Denmark) in a single layer. Harvested cells were seeded onto the dentin specimens (1 × 106 cells/plate) and cultured in normal growth medium. The dentin slices were randomly assigned to the groups shown in Figure 1 (n = 250 per group). Group 1 was treated by 5.25% NaOCl. In Group 2, 5.25% NaOCl and 1 mg/mL Ca(OH)2 treatments were followed by PBS washing. In Group 3, 5.25% NaOCl and 1 mg/mL Ca(OH)2 treatments were followed by 17% EDTA. In Group 4, 5.25% NaOCl and 1 mg/mL Ca(OH)2 treatments were followed by 17% EDTA and culture media for 24 hours. In Group 5, 5.25% NaOCl and 1 mg/mL Ca(OH)2 treatments were followed by instrumentation and 17% EDTA.
For Ca(OH)2, a 1,000 mg/mL concentration is needed to create a pasty slurry similar to the mixture used clinically. Therefore, Ca(OH)2 paste was first prepared as a thick slurry by mixing the 1,000 mg Ca(OH)2 powder with 1 mL distilled water and further diluted in media for 1 mg/mL.
After 7 days of cell culture, the dentin slices were removed and placed in new Nunclon cell culture dishes. Cells were detached with 0.25% trypsin/EDTA and 2 minutes of incubation in Thermo Steri-Cycle CO2 incubators (Forma Scientific Inc., Marietta, OH, USA) at 37℃ in 5% CO2 for trypsin activation. After 5 minutes of centrifugation, the cells were placed on new plates, and MTT (Sigma Chemical Co., St. Louis, MO, USA) assay was performed to assess cell viability. The absorbance at 570 nm was measured using a spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA).
After 7 days of culture, cell attachment was also evaluated based on the expression levels of fibronectin-1 (FN-1) and secreted phosphoprotein-1 (SPP-1). Total cellular RNA was extracted from the DPSCs in each group using an RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA) according to the manufacturer's instructions. The RNA was treated with RNAse-free DNase set (Qiagen) during RNA extraction. The complementary DNA samples were prepared from the isolated RNA using a RT First Strand Kit (Qiagen) according to the manufacturer's instructions. qRT-PCR analysis was performed using ABI 7500 software (Applied Biosystems, Foster City, CA, USA) according to the standard protocol. The RT-PCR included 40 cycles of general denaturation at 94℃ (30 seconds), annealing, and elongation at 60℃ (45 seconds), except for the first cycle, which had a 15 minutes denaturation, and the last cycle, which had a 7 minutes elongation at 72℃. Real-time polymerase quantification of the signals was performed by normalizing the gene signals with β-actin signal. The primers used to assess FN-1 and SPP-1 expression levels are shown in Table 1.
After 7 days of culture, a dentin slice from each group was selected randomly. The slices were washed 3 times with PBS and fixed in 2% glutaraldehyde for 5 minutes. The slices were then dehydrated in a graded series of ethanol, dried with hexamethyldisilazane, and gold coated. Cell morphology was assessed in each group with a scanning electron microscope (HITACHI S-3000N, Hitachi Co., Tokyo, Japan).
To compare differential gene expression under various conditions, we performed qRT-PCR analyses of mineralization-related genes. The primers for the differentiation markers of mineralization are given in Table 2. All DPSCs were cultured in vitro at 37℃ in a humidified atmosphere of 5% CO2 for 28 days. The culture medium in all groups was replenished every 3 days. The procedures were the same as those for the cell attachment assay.
All experiments were repeated in at least triplicate under 3 independent conditions. All data were presented as means and standard deviations. The Mann-Whitney U test was used to determine the statistical differences between the experimental groups with SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Adjusted p values less than 0.05 were considered statistically significant.
The DPSCs showed elongated, spindle-shaped and typical fibroblast-like cell morphology. After assessment of the cells by flow cytometric analysis, we chose 11 cell lines conforming to the minimal criteria for mesenchymal stem cells. These selected 11 cell lines were mixed and used for the following studies. The results of flow cytometric analysis of markers for the 11 selected cell lines are shown in Table 3.
The cell viability of all groups was lower than that in the control. The cell viability in the groups 2 - 5 were statistically not different from that in the group 1 (Figure 2).
Gene expression levels of FN-1 and SPP-1 were compared after 4 days of culture. Compared with the gene expression levels in Group 2, those in Groups 3 - 5 were significantly higher (p = 0.037, Figure 3).
The DPSCs in Group 1 did not attach to the dentin surfaces. However, the cells in Groups 2 - 5 were attached to the dentin surface. The cells were elongated and had longer cytoplasmic processes in Groups 2 - 5 (Figure 4). In particular, the dentin surfaces of the Group 5 samples were overlapped by proliferated cell layers (Figure 4d).
The gene expression levels of dentin matrix protein-1 (DMP-1) and dentin sialophosphoprotein (DSPP) were compared among the 5 groups after 4 weeks of culture. Cells in the control group were cultured in differentiation media. The gene expression level of DMP-1 was significantly higher in Groups 2 - 5 than in the control (p < 0.05). The DSPP level was significantly higher in Groups 3 - 5 than in the control (p < 0.05), but DMP-1 and DSPP expression levels were not significantly different between Groups 4 and 5 (Figure 5).
The present study evaluated the effect of sequential application of NaOCl and Ca(OH)2 on the attachment and differentiation of DPSCs using an experimental setting similar to a clinical trial. Regenerative endodontics in clinical practice does not produce a smear layer, as it minimizes instrumentation within root canals to protect the thin root dentin wall of immature permanent teeth. The smear layer is 1 to 5 µm thick denatured cutting debris produced on instrumented dentin surfaces. It is composed of dentin, odontoblastic processes, nonspecific inorganic contaminants, and microorganisms.181920 The existence of a smear layer in regenerative endodontics is a critical factor that may be responsible for surgical failure, as it interrupts DPSC adhesion.21 Therefore, unlike previous research, this study involved the sterilization of dentin specimens with ethylene oxide gas and EDTA treatment to remove the smear layer produced during the preparation of the dentin specimens.17 All concentration of Ca(OH)2 promoted stem cells from apical papilla survival, especially the concentration of 1 mg/mL, a level at which the survival rate of stem cells from apical papilla was significantly higher according to Ruparel et al.16
First, MTT assay was performed to assess the cell survival rate in each group. The MTT assay indicated greater cell viability in Groups 2 - 5 compared with that in Group 1. Unlike the latter, the former groups underwent a 7 day Ca(OH)2 treatment after NaOCl pretreatment. The high cell viability in Groups 2 - 5, which underwent additional treatment processes after Ca(OH)2 treatment, suggested that Ca(OH)2 had positive effects on cell survival. The highly alkaline pH of Ca(OH)2 encouraged the survival, migration, and proliferation of DPSCs, aiding the formation of reparative dentin.151622 Therefore, we concluded that Ca(OH)2 played a decisive role in boosting cell viability. The SEM images showed that the cells in Groups 2 - 5 adhered successfully, as opposed to those in Group 1, which failed. Within 7 days, the cells had stretched and had long cytoplastic processes. These results matched those of the MTT assays. In particular, Ca(OH)2 treatment enhanced cell adhesion and proliferation. Secondarily, EDTA and the instrumentation used during the removal of Ca(OH)2 changed the properties of the dentin surface, thereby creating an environment that facilitated DPSC adhesion.
Next, cell attachment was assessed via adhesion molecule qRT-PCR with FN-1 and SPP-1. FN-1 was an extracellular matrix molecule important in cell adhesion, and SPP-1 was an abundant noncollagenous bone matrix protein that played a role in bone-cell attachment.2324 The MTT results and SEM images confirmed that few, if any, living cells were present in Group 1. Thus, this group was excluded from qRT-PCR analysis. FN-1 and SPP-1 gene expression levels for Group 2, for which NaOCl and Ca(OH)2 treatments were followed by PBS washing, were significantly different from those of Groups 3 - 5. The high expression of FN-1 and SPP-1 in Groups 3 - 5 meant that cell attachment was also increased. EDTA played a role in cell adhesion and odontoblast differentiation by changing dentin surfaces, and the significant difference between Groups 2 and 3 indicated that EDTA additionally decalcified the dentin surface and increased wettability.17 This result was similar to that of a previous study describing changes in the dentin surfaces caused by the chelating effect of EDTA.25 The results for Groups 2 and 4 illustrated that EDTA initially created an environment in which cells could adhere, after which FBS neutralized the cell toxicity of NaOCl, thus increasing cell adhesion. FBS diluted or neutralized toxic irrigants and helped protect living cells.26
The use of instrumentation, which was part of the treatment for Groups 2 and 5, could effectively remove any remaining Ca(OH)2. Although Ca(OH)2 was commonly used in a variety of clinical situations as an antiseptic for root canal treatment, imperfectly removed Ca(OH)2 could block sealer penetration into dentinal tubules and increased apical leakage, resulting in failed root canals.27 Therefore, Ca(OH)2 applied to the root canal should be thoroughly removed for complete canal sealing. An experiment by Kenee et al. showed that when compared with an instrument and an irrigant, rotary, and ultrasonic instrumentation techniques were more effective in removing Ca(OH)2.28 Thus, instrumentation was standardized at the size 40/0.04 taper profile at 300 rpm, and scraping of the entire dentin surface was performed once in the present study. The removal of Ca(OH)2 via instrumentation was followed by the decalcification of the dentin surfaces using EDTA, which exposed the dentinal tubule and collagen fiber, creating an environment in which stem cells could easily adhere.
Group 1, which contained few living cells, was excluded from cell differentiation analysis as it was from the cell attachment qRT-PCR evaluation. However, as part of this analysis, the control group underwent a cell culture assay in differentiation media without dentin samples. DMP-1, an essential noncollagenous and acidic phosphorylated extracellular matrix protein, was highly expressed in odontoblasts. DMP-1 played a primary role in dentin mineralization.29 DSPP played a key role in the regulation of mineral deposition.30 DMP-1 was expressed at higher levels in Groups 2 - 5 than the control group, whereas DSPP showed higher expression levels in Groups 3 - 5. The significantly higher DMP-1 and DSPP expression levels could be interpreted as showing that after the NaOCl treatment, Ca(OH)2 treatment triggered odontogenesis, leading to odontoblast differentiation. In particular, the lack of a significant difference in expression levels between Groups 4 and 5 demonstrated that there was no difference between the technique using FBS for 24 hours as a neutralizing treatment agent for NaOCl and that in which instrumentation was performed followed by NaOCl and Ca(OH)2 treatments. In other words, creating an environment to facilitate better stem cell attachment and differentiation in the root canal through instrumentation and EDTA treatment was more effective than the cumbersome process of using FBS for 24 hours to neutralize NaOCl.
In this study, Ca(OH)2 improved the survival and attachment of cells after NaOCl treatment. Ca(OH)2 treatment was believed to have caused a favorable change in cell attachment and differentiation on the dentin surface. Possible mechanism included the release of a growth factor from dentin by Ca(OH)2 that enhanced cell attachment and differentiation.31 If Ca(OH)2 treatment improved the biochemical environments, and EDTA or instrumentation improved the biomechanical environments, the survival and differentiation of stem cells were expected to increase.
This present research was an in vitro study that preceded transplantation experiments, which were currently underway in large animals to investigate methods for achieving the complete regeneration of pulp. The results of this study could be used as the basis for in vivo experiments. Regenerative endodontics in clinical practice involves intracanal medication after sequential use of an irrigant in an infected root canal. Previous studies had examined NaOCl and Ca(OH)2 treatments independently. This study is significant in that it integrated these treatments, creating an environment closer to that found in actual clinical practice. However, due to the in vitro limitations of this study, additional in vivo studies are necessary. Furthermore, a long-term study is required, as the PCR results for the attachment and differentiation factors are measured at specific points and are therefore limited. In addition, studies of a counteragent to neutralize the toxicity of NaOCl and research on scaffolding and growth factors for enhanced tissue engineering are needed. If the technique elaborated in this study can be successfully performed in regenerative endodontics for immature permanent teeth in clinical practice, it will be proven to be applicable to endodontics for mature permanent teeth as well. Ultimately, the goal of regenerative endodontics will be met through complete pulp-dentin complex regeneration.
Figures and Tables
 | Figure 1Experimental groups for the attachment and differentiation of dental pulp stem cells. Cell attachment analysis with 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (tetrazolium-based colorimetric assay). |
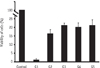 | Figure 2Cell viability analysis with MTT assay. Cells in the control group were grown on plates. Data were obtained from 3 separate experiments. MTT assay, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl-2H-tetrazolium bromide assay. |
 | Figure 3Expression levels of (a) fibronectin-1 (FN-1), and (b) secreted phosphoprotein-1 (SPP-1). Data were obtained from 3 separate experiments. *Mann-Whitney U test, level of statistical significance was set at p < 0.05. |
 | Figure 4Scanning electron microscopic views of dental pulp stem cell morphology on dentin surfaces after 7 days of culture. (a) group 2; (b) group 3; (c) group 4; (d) group 5 (magnification, ×1,000). |
 | Figure 5Expression levels of (a) dentin matrix protein-1 (DMP-1), and (b) dentin sialophosphoprotein (DSPP). Data were obtained from 3 separate experiments. *Mann-Whitney U test, level of statistical significance was set at p < 0.05. |
Table 1
Specific primer sets targeted for cell adhesion markers

| Gene name | Direction | Sequence |
|---|---|---|
| Fibronectin-1 | Forward | TCACAGACAGTGGTGTGGTC |
| Reverse | TCCTGCCCATTGTAGGTGAAT | |
| Secreted phopsphoprotein-1 | Forward | CAGCAACCGAAGTTTTCACT |
| Reverse | GCATCAGGGTACTGGATGTC |
Table 2
Specific primer sets targeted for odontogenic differentiation markers

| Gene name | Direction | Sequence |
|---|---|---|
| Dentin matrix protein-1 | Forward | CCCAAGATACCACCAGTGAG |
| Reverse | CACCCAGTGCTCTTCACTCT | |
| Dentin sialophosphoprotein | Forward | TTAAATGCCAGTGGAACCAT |
| Reverse | ATTCCCTTCTCCCTTGTGAC |
Table 3
Growth characteristics and immunophenotypic characterization of established cell lines

Acknowledgment
This research was supported by Basic Science Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A3013465).
References
1. Jung IY, Lee SJ, Hargreaves KM. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. J Endod. 2008; 34:876–887.

2. Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod. 2009; 35:1343–1349.

3. Ding RY, Cheung GS, Chen J, Yin XZ, Wang QQ, Zhang CF. Pulp revascularization of immature teeth with apical periodontitis: a clinical study. J Endod. 2009; 35:745–749.

4. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004; 30:196–200.

5. Thibodeau B, Trope M. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr Dent. 2007; 29:47–50.
6. Martin G, Ricucci D, Gibbs JL, Lin LM. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J Endod. 2013; 39:138–144.

7. Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, Diogenes A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011; 37:1109–1115.

8. Wennberg A. Biological evaluation of root canal antiseptics using in vitro and in vivo methods. Scand J Dent Res. 1980; 88:46–52.

9. Chang YC, Huang FM, Tai KW, Chou MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 92:446–450.

10. Heling I, Rotstein I, Dinur T, Szwec-Levine Y, Steinberg D. Bactericidal and cytotoxic effects of sodium hypochlorite and sodium dichloroisocyanurate solutions in vitro. J Endod. 2001; 27:278–280.

11. Ring KC, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. The comparison of the effect of endodontic irrigation on cell adherence to root canal dentin. J Endod. 2008; 34:1474–1479.

12. Shah N, Logani A, Bhaskar U, Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. J Endod. 2008; 34:919–925. Discussion 1157

13. Hoshino E, Kurihara-Ando N, Sato I, Uematsu H, Sato M, Kota K, Iwaku M. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J. 1996; 29:125–130.

14. Chueh LH, Ho YC, Kuo TC, Lai WH, Chen YH, Chiang CP. Regenerative endodontic treatment for necrotic immature permanent teeth. J Endod. 2009; 35:160–164.

15. Althumairy RI, Teixeira FB, Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod. 2014; 40:521–525.

16. Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012; 38:1372–1375.

17. Pang NS, Lee SJ, Kim E, Shin DM, Cho SW, Park W, Zhang X, Jung IY. Effect of EDTA on attachment and differentiation of dental pulp stem cells. J Endod. 2014; 40:811–817.

18. Brännström M. Smear layer: pathological and treatment considerations. Oper Dent Suppl. 1984; 3:35–42.
19. Czonstkowsky M, Wilson EG, Holstein FA. The smear layer in endodontics. Dent Clin North Am. 1990; 34:13–25.
20. Takeda FH, Harashima T, Kimura Y, Matsumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of laser. Int Endod J. 1999; 32:32–39.

21. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:658–666.

22. Guven EP, Yalvac ME, Sahin F, Yazici MM, Rizvanov AA, Bayirli G. Effect of dental materials calcium hydroxide-containing cement, mineral trioxide aggregate, and enamel matrix derivative on proliferation and differentiation of human tooth germ stem cells. J Endod. 2011; 37:650–656.

23. Kapila YL, Lancero H, Johnson PW. The response of periodontal ligament cells to fibronectin. J Periodontol. 1998; 69:1008–1019.

24. Noda M, Vogel RL, Craig AM, Prahl J, DeLuca HF, Denhardt DT. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990; 87:9995–9999.

25. Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007; 6:997–1003.

26. Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin-a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res. 1985; 160:245–258.

27. Calt S, Serper A. Dentinal tubule penetration of root canal sealers after root canal dressing with calcium hydroxide. J Endod. 1999; 25:431–433.

28. Kenee DM, Allemang JD, Johnson JD, Hellstein J, Nichol BK. A quantitative assessment of efficacy of various calcium hydroxide removal techniques. J Endod. 2006; 32:563–565.

29. Yue J, Wu B, Gao J, Huang X, Li C, Ma D, Fang F. DMP1 is a target of let-7 in dental pulp cells. Int J Mol Med. 2012; 30:295–301.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download