Abstract
Objectives
The use of chitosan nanoparticles (CNPs) in endodontics is of interest due to their antibiofilm properties. This study was to investigate the ability of bioactive CNPs to remove the smear layer and inhibit bacterial recolonization on dentin.
Materials and Methods
One hundred bovine dentin sections were divided into five groups (n = 20 per group) according to the treatment. The irrigating solutions used were 2.5% sodium hypochlorite (NaOCl) for 20 min, 17% ethylenediaminetetraacetic acid (EDTA) for 3 min and 1.29 mg/mL CNPs for 3 min. The samples were irrigated with either distilled water (control), NaOCl, NaOCl-EDTA, NaOCl-EDTA-CNPs or NaOCl-CNPs. After the treatment, half of the samples (n = 50) were used to assess the chelating effect of the solutions using portable scanning electronic microscopy, while the other half (n = 50) were infected intra-orally to examine the post-treatment bacterial biofilm forming capacity. The biovolume and cellular viability of the biofilms were analysed under confocal laser scanning microscopy. The Kappa test was performed for examiner calibration, and the non-parametric Kruskal-Wallis and Dunn tests (p < 0.05) were used for comparisons among the groups.
Bacterial biofilms are structured communities of cells adhered to an organic surface and stabilized in an extracellular matrix.1 The high density of bacterial cells in biofilm communities and the inherent resistance of biofilm bacteria to antimicrobials/host defence systems are the main factors responsible for most persistent and chronic bacterial infections.1 The combined use of mechanical instrumentation and chemical irrigation has been recommended to obtain effective elimination of biofilms from the root canal system.2 Among the commonly used endodontic irrigants, sodium hypochlorite (NaOCl) is the most recommended irrigant due to its substantial tissue dissolution capacity and antibacterial effect.3,4 However, NaOCl causes dentin collagen denaturation and dissolution. Consequently, the use of a high concentration of this solution for long periods of time may cause ultrastructural damage into the dentin.5 In addition, NaOCl is not capable of completely debriding the root canals or eliminating the biofilm bacteria.4 The chelating agents well remove smear layers from root dentin, thus are used for the final irrigation of the root canals.6 The smear layer is composed of very small particles that may be solubilized in acids.7 The most common chelating solutions contain ethylenediaminetetraacetic acid (EDTA), which reacts with the calcium ions in dentin and forms soluble calcium chelates.8 However, the chelating agents may alter the structural characteristic of the dentin resulting in a compromised mechanical integrity and an increased potential for bacterial adherence on the collagen.9,10
Chitosan is a non-toxic cationic biopolymer usually obtained by alkaline deacetylation from chitin, which is the principal component of crustacean exoskeletons.11 The covalent immobilization of chitosan on dentinal collagen has been proposed to induce the remineralization of the exposed and demineralized dentin structure because its functional phosphate groups might bind to calcium ions to form a favorable surface for crystal nucleation, resulting in the formation of a calcium phosphate layer.12 Chitosan treatment improves the resistance of the dentinal surface to degradation by collagenase.13 Furthermore, chitosan presents with biocompatibility, chelating capacity and also antimicrobial effects against a broad range of gram-positive and gram-negative bacteria as well as fungi.14,15,16,17
Previous in vitro studies have demonstrated the significant antibiofilm efficacy of chitosan nanoparticles (CNPs).14,15 However, testing the efficacy of these nanoparticles on biofilms formed in situ would provide a stronger correlation with the findings from the in vivo studies since coronal leakage of saliva is one of the main factors that allows for bacterial recolonization in root-filled teeth.18 In addition, there is limited information about the use of CNPs as a chelating agent.16,19 The current study aims to investigate the ability of CNPs to act as a final irrigant to remove the smear layer from a root-dentin surface and simultaneously inhibit bacterial recolonization/early biofilm formation when exposed to saliva.
The present study was performed according to the guidelines of the Institutional Research Human Ethics Committee (Protocol 166/2011). Eighty rectangular dentin blocks (5 mm × 5 mm × 3 mm) were obtained from bovine radicular dentin. Dentin debris were created on the sample surface by sectioning every side of the block. The sectioning procedures were performed using an Isomet saw (Buehler Ltd., Evanston, IL, USA). The dentin blocks were autoclaved for 30 minutes at 121℃ (Sercon-Modelo HS, Mogi das Cruzes, SP, Brazil). The samples were not treated with any chemicals before being autoclaved to maintain the dentin surface debris generated during the sectioning of these samples.
The CNPs were synthesized based on a previously published protocol using an ionic gelation method.20 Briefly, the chitosan powder (Polymar Ciência e Nutrição S/A, Fortaleza, Brazil) was dissolved in 1% acetic acid under magnetic stirring at room temperature. Next, 1 mg/mL sodium tripoly-phosphate solution (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) was added into the chitosan solution. The preparations were mixed with a Polytron homogenizer (PT-3000, Brinkman Instruments, Rexdale, ON, Canada) at 5,000 rpm with drop-wise addition of the tripoly-phosphate solution, thus achieving a final CNPs concentration of 1.29 mg/mL. The zone of opalescent suspension was visible with the formation of the nanoparticles, and the solution was further examined for nanoparticle characterization. The concentration of tripolyphosphate solution in the final solution was 0.3 - 0.6 mg/mL. The range of the nanoparticles' size was 85 - 221 nm.
The irrigating solutions used included 2.5% NaOCl for 20 minutes, 17% EDTA for 3 minutes and 1.29 mg/mL CNPs for 3 minutes. The NaOCl was replenished every 5 minutes to simulate clinical conditions. One hundred bovine dentin blocks were equally divided in five groups (n = 20 per group) and treated with either distilled water (control, group 1), NaOCl (group 2), NaOCl-EDTA (group 3), NaOCl-EDTA-CNPs (group 4), and NaOCl-CNPs (group 5). The volume was standardized to 3 mL for all of the solutions. The experiments were performed in 24 well tissue culture plates by immersing the dentin samples in the irrigant solutions. After the irrigation procedure was performed, half of the samples (n = 50) were used to analyse the chelating effect of the solutions using a portable scanning electronic microscope (PSEM, Aspex Corporation, Delmont, PA, USA), while the other half (n = 50) was infected intra-orally to assess bacterial biofilm forming capacity using a confocal laser scanning microscope (CLSM, Leica TCS-SPE, Mannheim, Germany). Therefore, the experiment was conducted in two assays, which were formed by five groups each containing 10 samples.
After the irrigation procedures, the samples were dried at room temperature for 24 hours and fixed on stubs. Three micrographs per block were analysed using a PSEM with a magnification of 500 times at 10 kV creating a total of 150 images. The area of each image represented 1,024 µm × 1,024 µm. For quantification purposes, the PSEM pictures were divided into 100 areas using a digital grid, and then examined by three endodontic specialists who assessed the amount of area in each grid that was covered by a smear layer. Each examiner evaluated the pictures twice, with an interval of one week between each observation. The values obtained by one examiner that showed concordance with that from the other examiners and had the lowest amount of discrepancy between these observations were chosen for the statistical analysis. A scores of one to four were ascribed to each sample according to a previously published methodology that was slightly modified.21 Having less than 10% of the area containing open dentinal tubules was scored as one, having 10 - 50% of the area containing open dentinal tubules was scored as two, having 50 - 70% of the area containing open dentinal tubules was scored as three and having more than 70% of the area containing open dentinal tubules was scored as four.
An in situ model was modified to simulate the presence of intra-oral dentinal biofilms.4 Twenty treated dentin blocks were fixed in the cavities of a Hawley orthodontic device using sticky wax (Kota Ind. e Com. Ltda., São Paulo, SP, Brazil). The procedure was repeated to complete the infection of all of the samples. To standardize the biofilm thickness as much as possible, one healthy volunteer used the orthodontic device for 48 hours. After this period, the samples were transferred to test tubes containing 5 mL of brain-heart infusion broth, and then incubated at 37℃ for 24 hours. The subject maintained a controlled routine of food and drink consumption while using the intra-oral device during the infection period except during regular oral-hygiene practices. The same diet was supplied during each infection period.
After the dentinal infection period, the samples were rinsed with sterile distilled water to remove the non-adherent cells and culture medium. The biofilms were stained with the Live/Dead BacLight Bacterial Viability kit (Invitrogen, Eugene, OR, USA) and analysed with a CLSM. Four stacks were analysed from random areas of each sample, totalling 200 images. Each block was scanned using a 40 times oil lens, 1.5 µm step-size and a format of 512 pixels × 512 pixels. The area of each image represented 275 µm × 275 µm. The parameters evaluated were the total biovolume expressed in µm3/µm2 in accordance with Heydorn et al. and the percentage of viable cells.22 The software Bioimage_L (http://bioimagel.com) was used to calculate these parameters.23 Representative images obtained from the PSEM (post-irrigation) and CLSM (post-infection) are shown in Figure 1. A previous pilot study has demonstrated that the residual effect of NaOCl did not interfere with the activities of the experimental solutions on the dentinal surface and bacterial biofilm.
All statistical analyses were performed with the Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). The Kappa test was utilized to determinate the concordance among the examiners (Kappa ≥ 0.75). The non-parametric Kruskal-Wallis and Dunn tests (p < 0.05) were used to perform multiple comparisons among the groups as the data did not show a normal distribution.
The results of the Kappa tests showed good interexaminer agreement with values of 0.8 or above for all of the different categories.
The smear layer was significantly reduced in the groups treated with NaOCl-EDTA, NaOCl-EDTA-CNPs and NaOCl-CNPs in comparison with that of the control and NaOCl groups (p < 0.05). The percentage of areas with or without observable dentinal tubules per score and the statistical differences among the groups are shown in the Figure 2.
The biovolume and bacterial viability were significantly lower in the samples receiving final irrigation with CNPs than those in the control were (p < 0.05). The NaOCl-EDTA-CNPs and NaOCl-CNPs groups were not statistically different (p > 0.05). The bacterial colonization and cell viability in the NaOCl and NaOCl-EDTA groups were not statically different than those in the control group (p > 0.05). The comparison of the medians and 25 - 75% percentiles of the total biovolume and the percentage of live cells among all of the groups are shown in Table 1.
The bovine dentin was chosen as a substrate in the present study because it is a suitable substitute for human dentin when testing for erosion/abrasion, which is closely related with smear layer removal.24 In addition, the topography of the substrate is an important factor when discussing the survival of biofilms since rough surfaces will increase bacterial adhesion and retention providing anchor points for microorganisms and their nutrients.25 Thus, it should be logical to assume that the bovine dentin facilitate bacterial invasion due to the significantly greater tubular diameter when compared with that of human dentinal tubules.26 However, these factors do not substantially affect studies focused on evaluating the bacterial volume and cell viability of mature biofilms localized from the dentinal surface to the highest part of the biofilm.4,27,28
The chelating capacity of chitosan on root canal dentin has been assessed by only two previous studies, which showed that the irrigation of the root canals with a CNPs-based solution for 3 minutes effectively removed the smear layer from the root canals.16,19 These results were in accordance with those obtained in the present study, where the final irrigation with CNPs for 3 minutes effectively removed the inorganic contents from the dentin. Even though all the EDTA- and CNPs-treated experimental groups showed similar efficacy in removing the smear layer, the use of CNPs after irrigation with NaOCl is recommended because of the ability of the CNPs on dentin to resist bacterial adherence and early biofilm formation.15 This resistance is an important advantage of using CNPs over EDTA, which presented a similar chelating effect.
The chelating effect of CNPs and EDTA could cause dentin demineralization.16 However, a previous study has shown that dentin surfaces coated with chitosan have the potential to remineralize the demineralized dentin.12 The results of the present study suggest that the chelating effect of CNPs irrigation following 17% EDTA is similar to the effect caused by each of these solutions when they are used independently. This observation could be explained by the finding that the chelating ability of the polymeric chitosan matrices was maintained when it was modified with the chemical immobilization of EDTA.29
The chelating mechanism of chitosan on dentin has previously not been well documented. However, this bioactive biopolymer is widely used as a chelating agent to absorb heavy metals from wastewater.30 Two theories have been used to explain the chelating mechanism of chitosan. First, the bridge model states that two or more amino groups of chitosan bind to the same metal ion.31 Second, the pendant model suggests that one amino group is utilized in the binding, and the metal ion is linked to the amino group like a pendant.32 Either of the two mechanisms could be responsible for the chelation of calcium ions in dentin resulting in the depletion of inorganic matter from the smear layer.33
In the present study, the bacterial infection was created in situ because biofilm development in a natural environment has been demonstrated to be profoundly different from the biofilm formed in vitro.34 Many factors have been associated with failures in endodontic treatment. Yet, bacterial re-entry and re-contamination of the root canal by coronal microleakage subsequent to root-filling, is suggested to be one of most important causes for endodontic treatment failure.35 In addition to salivary microleakage, the filled root canals may be exposed to the oral environment in certain situations such as during the loss or fracture of dental structures/restorations as well as during intraradicular preparations for prosthetic purposes, thereby allowing the more than 175 bacterial species present in human saliva to invade the root canal spaces.36,37 It is important to note that intracanal salivary penetration via coronal leakage in endodontically treated teeth can occur in only 2 - 3 days.38,39
In this study, the bacterial volume and cell viability were found to be highest when the samples were irrigated with NaOCl and NaOCl-EDTA. EDTA has been reported as a weak antibacterial agent, and its effect against gram-positive bacteria has been found to be almost nil.40 EDTA has shown selectivity for gram-negative bacteria and is known to destabilize the bacterial cell membrane and cause release of lipopolysaccharides. In contrast, the biofilm formed after treatment with NaOCl-EDTA-CNPs and NaOCl-CNPs were significantly lower than that in the control and NaOCl-EDTA groups. These results corroborate the theory that chitosan has the ability to interfere with bacterial adhesion thereby hindering the biofilm formation, and that EDTA has the power to alter the physicochemical properties of dentin that contribute to the bacterial adhesion.10,15
The antibacterial mechanism of chitosan has been attributed to its polycationic nature that interacts with the negatively charged surface of bacteria, altering cell permeability and resulting in the leakage of intracellular components.41,42 In addition, CNPs are able to inhibit bacterial enzymatic degradation reducing the possibility of bacterial penetration and dentinal micro-fractures.43 This biopolymer is also capable of improving the mechanical properties of the root dentin.13 Grande et al. showed that the reaction between NaOCl and EDTA led a very slow degradation of the acid.44 Although degradation of EDTA was progressive, its properties were not affected in periods of time clinically realistic. In addition, the structure and molecular weight of chitosan have been found to be affected by oxidation caused by NaOCl.45 However, more detailed evidence on the interaction of NaOCl with chitosan applicable to root canal disinfection is required.
Acknowledgement
This study was supported by the São Paulo Research Foundation (FAPESP 2011/08184-8 and 2012/06738-9) and the National Counsel of Technological and Scientific Development (CNPq).
References
1. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999; 284:1318–1322. PMID: 10334980.

2. Siqueira JF Jr, Paiva SS, Rôças IN. Reduction in the cultivable bacterial populations in infected root canals by a chlorhexidine-based antimicrobial protocol. J Endod. 2007; 33:541–547. PMID: 17437868.

3. Pascon FM, Kantovitz KR, Sacramento PA, Nobredos-Santos M, Puppin-Rontani RM. Effect of sodium hypochlorite on dentin mechanical properties. A review. J Dent. 2009; 37:903–908. PMID: 19665276.
4. Del Carpio-Perochena AE, Bramante CM, Duarte MA, Cavenago BC, Villas-Boas MH, Graeff MS, Bernardineli N, de Andrade FB, Ordinola-Zapata R. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J Endod. 2011; 37:1134–1138. PMID: 21763908.

5. Zhang K, Kim YK, Cadenaro M, Bryan TE, Sidow SJ, Loushine RJ, Ling JQ, Pashley DH, Tay FR. Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. J Endod. 2010; 36:105–109. PMID: 20003945.

6. Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002; 28:17–19. PMID: 11806642.

7. Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:658–666. PMID: 12464887.

8. Violich DR, Chandler NP. The smear layer in endodontics - a review. Int Endod J. 2010; 43:2–15. PMID: 20002799.

9. Garberoglio R, Becce C. Smear layer removal by root canal irrigants. A comparative scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1994; 78:359–367. PMID: 7970599.
10. Kishen A, Sum CP, Mathew S, Lim CT. Influence of irrigation regimens on the adherence of Enterococcus faecalis to root canal dentin. J Endod. 2008; 34:850–854. PMID: 18570994.

11. Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, Dhawan S. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004; 274:1–33. PMID: 15072779.

12. Xu Z, Neoh KG, Lin CC, Kishen A. Biomimetic deposition of calcium phosphate minerals on the surface of partially demineralized dentin modified with phosphorylated chitosan. J Biomed Mater Res B Appl Biomater. 2011; 98:150–159. PMID: 21538842.
13. Shrestha A, Friedman S, Kishen A. Photodynamically crosslinked and chitosan-incorporated dentin collagen. J Dent Res. 2011; 90:1346–1351. PMID: 21911787.

14. No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol. 2002; 74:65–72. PMID: 11929171.

15. Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod. 2008; 34:1515–1520. PMID: 19026885.

16. Silva PV, Guedes DF, Nakadi FV, Pécora JD, Cruz-Filho AM. Chitosan: a new solution for removal of smear layer after root canal instrumentation. Int Endod J. 2013; 46:332–338. PMID: 22970844.

17. Calamari SE, Bojanich MA, Barembaum SR, Berdicevski N, Azcurra AI. Antifungal and post-antifungal effects of chlorhexidine, fluconazole, chitosan and its combinations on Candida albicans. Med Oral Patol Oral Cir Bucal. 2011; 16:e23–e28. PMID: 20711160.

18. Magura ME, Kafrawy AH, Brown CE Jr, Newton CW. Human saliva coronal microleakage in obturated root canals: an in vitro study. J Endod. 1991; 17:324–331. PMID: 1779218.
19. Silva PV, Guedes DF, Pécora JD, da Cruz-Filho AM. Time-dependent effects of chitosan on dentin structures. Braz Dent J. 2012; 23:357–361. PMID: 23207849.

20. De Moura MR, Aouada FA, Avena-Bustillos RJ, McHugh TH, Krochta JM, Mattoso LHC. Improved barrier and mechanical properties of novel hydroxypropyl methylcellulose edible films with chitosan/tripolyphosphate nanoparticles. J Food Eng. 2009; 92:448–453.

21. Zandim DL, Corrêa FO, Rossa Júnior C, Sampaio JE. In vitro evaluation of the effect of natural orange juices on dentin morphology. Braz Oral Res. 2008; 22:176–183. PMID: 18622489.
22. Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000; 146:2395–2407. PMID: 11021916.

23. Chávez de Paz LE. Image analysis software based on color segmentation for characterization of viability and physiological activity of biofilms. Appl Environ Microbiol. 2009; 75:1734–1739. PMID: 19139239.

24. Wegehaupt F, Gries D, Wiegand A, Attin T. Is bovine dentin an appropriate substitute for human dentin in erosion/abrasion tests? J Oral Rehabil. 2008; 35:390–394. PMID: 18405276.
25. Whitehead KA, Rogers D, Colligon J, Wright C, Verran J. Use of the atomic force microscope to determine the effect of substratum surface topography on the ease of bacterial removal. Colloids Surf B Biointerfaces. 2006; 51:44–53. PMID: 16822658.

26. Lopes MB, Sinhoreti MA, Gonini Júnior A, Consani S, McCabe JF. Comparative study of tubular diameter and quantity for human and bovine dentin at different depths. Braz Dent J. 2009; 20:279–283. PMID: 20069249.

27. Del Carpio-Perochena A, Bramante CM, Hungaro Duarte MA, de Andrade FB, Cavenago BC, Villas-Bôas MH, Ordinola-Zapata R, Amoroso-Silva P. Application of laser scanning microscopy for the analysis of oral biofilm dissolution by different endodontic irrigants. Dent Res J (Isfahan). 2014; 11:442–447. PMID: 25225556.
28. Ordinola-Zapata R, Bramante CM, Cavenago B, Graeff MS, Gomes de Moraes I, Marciano M, Duarte MA. Antimicrobial effect of endodontic solutions used as final irrigants on a dentin biofilm model. Int Endod J. 2012; 45:162–168. PMID: 21985189.
29. Inoue K, Yoshizuka K, Ohto K. Adsorptive separation of some metal ions by complexing agent types of chemically modified chitosan. Anal Chim Acta. 1999; 388:209–218.

30. Bassi R, Prasher SO, Simpson BK. Effects of organic acids on the adsorption of heavy metal ions by chitosan flakes. J Environ Sci Health. 1999; 34:289–294.

31. Blair HS, Ho TC. Studies in the adsorption and diffusion of ions in chitosan. J Chem Technol Biotechnol. 1981; 31:6–10.

32. Vold IMN, Vårum KM, Guibal E, Smidsrød O. Binding of ions to chitosan-selectivity studies. Carbohydr Polym. 2003; 54:471–477.

33. Pimenta JA, Zaparolli D, Pécora JD, Cruz-Filho AM. Chitosan: effect of a new chelating agent on the microhardness of root dentin. Braz Dent J. 2012; 23:212–217. PMID: 22814688.

34. Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987; 41:435–464. PMID: 3318676.

35. Wang QQ, Zhang CF, Chu CH, Zhu XF. Prevalence of Enterococcus faecalis in saliva and filled root canals of teeth associated with apical periodontitis. Int J Oral Sci. 2012; 4:19–23. PMID: 22422085.

36. Saunders WP, Saunders EM. Coronal leakage as a cause of failure in root-canal therapy: a review. Endod Dent Traumatol. 1994; 10:105–108. PMID: 7995237.

37. Hasan NA, Young BA, Minard-Smith AT, Saeed K, Li H, Heizer EM, McMillan NJ, Isom R, Abdullah AS, Bornman DM, Faith SA, Choi SY, Dickens ML, Cebula TA, Colwell RR. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS One. 2014; 9:e97699. PMID: 24846174.

38. Madison S, Swanson K, Chiles SA. An evaluation of coronal microleakage in endodontically treated teeth. Part II. Sealer types. J Endod. 1987; 13:109–112. PMID: 3471832.

39. Madison S, Wilcox LR. An evaluation of coronal microleakage in endodontically treated teeth. Part III. In vivo study. J Endod. 1988; 14:455–458. PMID: 3273315.
40. Gray GW, Wilkinson SG. The effect of ethylenediaminetetra-acetic acid on cell walls of some gram-negative bacteria. J Gen Microbiol. 1965; 39:385–399. PMID: 4955831.
41. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003; 4:1457–1465. PMID: 14606868.

42. Young DH, Köhle H, Kauss H. Effect of chitosan on membrane-permeability of suspension-cultured glycine-max and phaseolus-vulgaris cells. Plant Physiol. 1982; 70:1449–1454. PMID: 16662696.
43. Persadmehr A, Torneck CD, Cvitkovitch DG, Pinto V, Talior I, Kazembe M, Shrestha S, McCulloch CA, Kishen A. Bioactive chitosan nanoparticles and photodynamic therapy inhibit collagen degradation in vitro. J Endod. 2014; 40:703–709. PMID: 24767568.
44. Grande NM, Plotino G, Falanga A, Pomponi M, Somma F. Interaction between EDTA and sodium hypochlorite: a nuclear magnetic resonance analysis. J Endod. 2006; 32:460–464. PMID: 16631849.

45. Yoo SH, Lee JS, Park SY, Kim YS, Chang PS, Lee HG. Effects of selective oxidation of chitosan on physical and biological properties. Int J Biol Macromol. 2005; 35:27–31. PMID: 15769512.

Figure 1
Representative images from the portable scanning electron microscope (×500) and confocal laser scanning microscope (×40). The irrigated pre-infection samples can be seen in images (a) - (e), and the infected samples after experimental irrigation protocols can be seen in images (f) - (j). A substantial amount of smear layer was observed when the samples were irrigated with sterile distilled water (a) and NaOCl (b). Visible dentinal tubules were seen in the samples treated with NaOCl-EDTA (c), NaOCl-EDTA-CNPs (d) and NaOCl-CNPs (e). A positive cellular viability and evident biomass were observed in the control (f), NaOCl (g) and NaOCl-EDTA (h) groups. The NaOCl-EDTA-CNPs and NaOCl-CNPs groups had decreased biomass (i) and interfered with bacterial growth (j). All bars represent 20 µm.
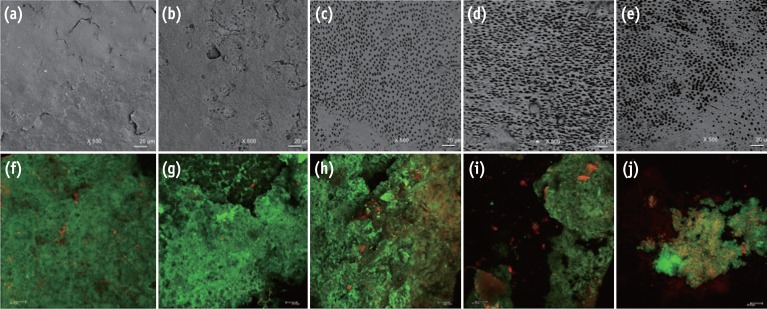
Figure 2
The percentage of areas with open dentinal tubules for each score (1 to 4). Having less than 10% of the area containing open dentinal tubules was scored as one, having 10 - 50% of the area containing open dentinal tubules was scored as two, having 50 - 70% of the area containing open dentinal tubules was scored as three and having more than 70% of the area containing open dentinal tubules was scored as four.
*Different letters within a column depict a significant difference (p < 0.05).
NaOCl, Sodium hypochlorite; NaOCl-EDTA, Sodium hypochlorite-ethylenediaminetetraacetic acid; NaOCl-EDTA-CNPs, Sodium hypochloriteethylenediaminetetraacetic acid-chitosan nanoparticles; NaOCl-CNPs, Sodium hypochlorite-chitosan nanoparticles.
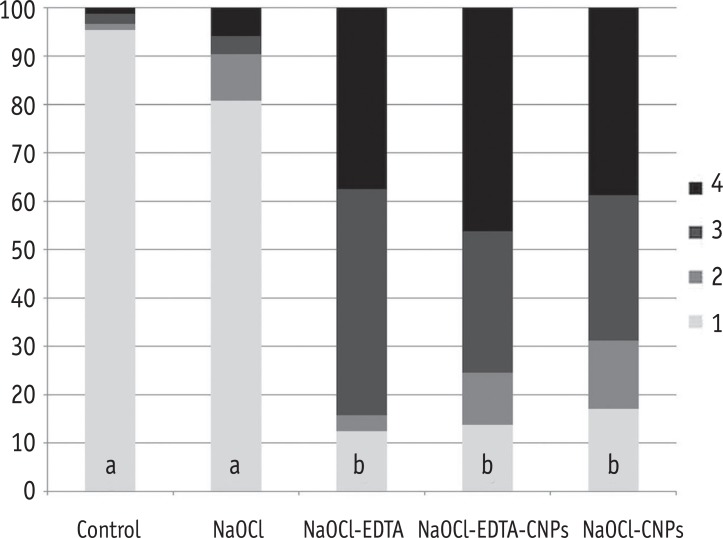
Table 1
Medians (25 - 75 percentiles) of the total biovolume and the percentage of live cells of the comparisons among the groups
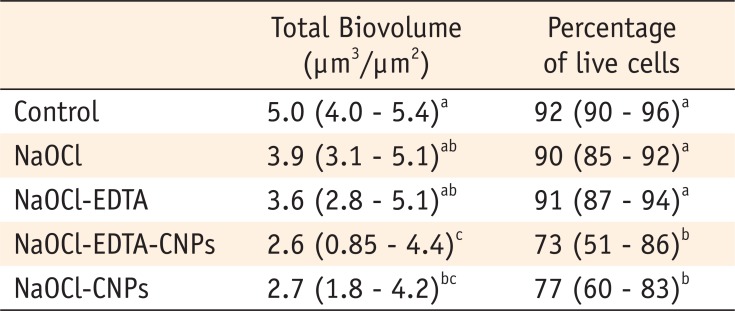
*Different superscript letters in each column represent significant differences (p < 0.05).
NaOCl, Sodium hypochlorite; NaOCl-EDTA, Sodium hypochlorite-ethylenediaminetetraacetic acid; NaOCl-EDTA-CNPs, Sodium hypochlorite-ethylenediaminetetraacetic acid-chitosan nanoparticles; NaOCl-CNPs, Sodium hypochlorite-chitosan nanoparticles.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download