Abstract
Objectives
This study evaluated the effect of lactic acid and acetic acid on the microhardness of a silorane-based composite compared to two methacrylate-based composite resins.
Materials and Methods
Thirty disc-shaped specimens each were fabricated of Filtek P90, Filtek Z250 and Filtek Z350XT. After measuring of Vickers microhardness, they were randomly divided into 3 subgroups (n = 10) and immersed in lactic acid, acetic acid or distilled water. Microhardness was measured after 48 hr and 7 day of immersion. Data were analyzed using repeated measures ANOVA (p < 0.05). The surfaces of two additional specimens were evaluated using a scanning electron microscope (SEM) before and after immersion.
Results
All groups showed a reduction in microhardness after 7 day of immersion (p < 0.001). At baseline and 7 day, the microhardness of Z250 was the greatest, followed by Z350 and P90 (p < 0.001). At 48 hr, the microhardness values of Z250 and Z350 were greater than P90 (p < 0.001 for both), but those of Z250 and Z350 were not significantly different (p = 0.095). Also, the effect of storage media on microhardness was not significant at baseline, but significant at 48 hr and after 7 day (p = 0.001 and p < 0.001, respectively). Lactic acid had the greatest effect.
Use of resin-based restorative dental materials has greatly increased in the recent years due to their optimal esthetics, enhanced properties, easy handling and the ability to optimally bond to tooth structure.1 The main drawback of composite resins is their polymerization shrinkage and the resultant stress that can lead to gap formation at the tooth-restoration interface, microleakage, hypersensitivity, pulp irritation, marginal discoloration and recurrent caries.23 Low-shrinkage silorane-based composites were introduced to overcome these shortcomings. They have low polymerization shrinkage due to the ring-opening polymerization mechanism of oxirane molecule.3
Restorative materials should have adequate longevity in order to be considered clinically successful.4 The survival of composite restorations depends not only on their innate characteristics, but also on the surrounding environment.56 Composite materials are more susceptible to chemical degradation than metal or ceramics due to the possession of organic matrix.7 Oral cavity is a complex aqueous environment where dental restorative materials are exposed to several factors namely saliva and low pH due to the consumption of acidic foods and release of organic acids in the dental biofilm. These conditions have a destructive effect on the polymer network affecting its physical and chemical properties in short-term or long-term.8
Numerous studies have evaluated water sorption, solubility and mechanical properties of composites after immersion in water, artificial saliva or ethanol in order to better understand the process of composite degradation.91011 Hardness is an important characteristic of restorative materials correlated with their intraoral compressive strength and resistance to softening.12 Low surface hardness is strongly correlated with insufficient wear resistance and susceptibility to scratching. It can also compromise fatigue strength and lead to restoration fracture.5 Dental biofilm contains high concentrations of lactic acid, acetic acid and propionic acid.1314 Previous studies have indicated that accumulation of dental biofilm does not depend on the oral hygiene or technique of plaque removal by the patients.15 Everyone can have the potential of producing organic acids in dental biofilm.16 It has been reported that low pH may affect the surface hardness of resin-based composites.17
To date, limited studies have investigated the effect of organic acids present in dental biofilm on methacrylate-based composites.1118 On the other hand, it has been claimed that silorane-based composites are less soluble due to the presence of siloxane molecules.19 However, to the best of our knowledge, no study has evaluated the effect of these acids on the surface hardness of silorane-based composites. Thus, this study aimed to assess the effect of lactic acid, acetic acid and distilled water on microhardness of a silorane-based compared to two methacrylate-based (nanofilled and microhybrid) composites. The null hypotheses were that type of composite would have no effect on the microhardness and that type of storage media would have no effect on microhardness.
The brand names, composition and the manufacturing company of the composites used in this study are shown in Table 1.
First, a stainless steel mold, 10 mm in diameter and 2 mm in thickness, was placed on a glass slab. Composite resin was applied to the mold and another glass slab was placed over it to ensure surface smoothness and uniform thickness of specimens and also to prevent void formation. According to the manufacturer's instructions, composite specimens were cured at both sides for 20 seconds using LED light-curing unit (Valo, Ultradent, Products Inc., South Jordan, USA) with 1,000 mW/cm2 intensity and then polished with 1,200, 1,500, 2,000, 2,500, 3,000 and 5,000 grit abrasive papers. A total of 90 specimens were fabricated (30 of each composite). Samples were immersed in an ultrasonic bath containing water for 4 minutes followed by 24 hours of distilled water storage at 37℃ to allow completion of polymerization. Baseline microhardness was assessed using a Vickers microhardness tester.
Immediately after measuring the baseline microhardness, specimens in each composite group were randomly divided into 3 subgroups of 10 and coded. Subgroup 1 specimens were immersed in screw-top vials containing distilled water (pH = 7) as the control subgroup, subgroup 2 specimens were immersed in lactic acid (pH = 4, 0.01 M), and subgroup 3 into acetic acid (pH = 4, 0.01 M). The vials containing specimens were stored in an incubator at 37℃ for 7 days.
The microhardness of specimens was measured at baseline and 48 hours and 7 days after immersion using a digital microhardness tester (Vickers hardness testing machine, KB HardWin XL, KB Pruftechnik GmbH, Germany), and 100 g load was applied by the indenter of the Vickers machine for 30 seconds at room temperature. Three indentations with more than 1 mm distance from the disc margins were made at different surface areas and the mean microhardness was calculated using the microhardness values of the three indentations. For the calculation of Vickers microhardness number (HV), the lengths of the two diagonals of each indentation were measured and HV was calculated using the following formula,
where F is the load applied and d is the mean length of the two diagonals of each indentation.20
Two extra specimens were fabricated in each group and evaluated before and after 7 days immersion using a scanning electron microscope (SEM, KYKY SBC-12, Beijing, China). For this purpose, surface of specimens was completely dried and gold coated with a sputter coater. SEM analysis was then performed at a voltage of 20 kV with ×3,000 magnification.
Repeated measures ANOVA was used for the comparison of microhardness of different composite specimens before and after immersion in the respective media. The microhardness value at different time points was considered as the repeated factor and the media factor and type of composite were considered as the between subject factors. If the interaction was significant, two-way repeated ANOVA was applied for the comparison of microhardness of composite specimens at each time point separately for each medium, separately for each composite in different media and also for the comparison of microhardness changes based on the type of composite and storage medium. Data were analyzed using SPSS software (IBM SPSS statistics 18, SPSS Inc., Chicago, IL, USA). A p value < 0.05 was considered significant.
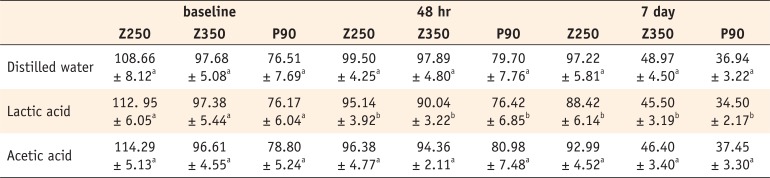
The mean microhardness values are shown in Table 2.
Repeated measures ANOVA revealed that the microhardness of all composite specimens decreased after 7 days of immersion (p < 0.001) and different composites changed variably in microhardness in different media (p < 0.001). At baseline, the interaction effect of type of composite and the media on the microhardness was not significant (Two-way ANOVA, p = 0.429), and we could show that the water immersion before baseline measurement after light curing did not have any effect on the microhardness. The microhardness values of the composites were significantly different (p < 0.001), that is, the microhardness of Z250 was higher than Z350 (p < 0.001) and the latter was higher than P90 (p < 0.001). The effect of media on microhardness was not significant (p = 0.346). At 48 hours after immersion, the interaction effect of type of composite and the media was not significant (p = 0.444). The microhardness values of the composites were significantly different (p < 0.001). The microhardness values of Z250 (p < 0.001) and Z350 (p < 0.001) were higher than that of P90. However, the microhardness values of Z250 and Z350 were not significantly different (p = 0.095). Also, the effect of the media on microhardness was significant (p = 0.001). The difference between lactic acid and distilled water (p = 0.001) and lactic acid and acetic acid (p = 0.043) in this respect was significant. However, distilled water and acetic acid had no significant difference in this regard (p = 0.403). At 7 days, the interaction effect of independent variables on microhardness was not significant (p = 0.111). The microhardness values of the composites were significantly different (p < 0.001). The microhardness of Z250 was higher than Z350 (p < 0.001) and the latter was higher than P90 (p < 0.001). The effect of media on microhardness was significant as well (p < 0.001). The differences between distilled water and lactic acid (p < 0.001) and lactic acid and acetic acid (p = 0.031) were significant in this respect, whereas distilled water had no significant difference with acetic acid (p = 0.138, Table 1).
Composites compared in our study were all manufactured by 3M ESPE. P90 is a silorane-based and Z250 and Z350 are methacrylate-based composites with similar resin base (bisphenylglycidyl dimethacrylate [Bis-GMA]; ethoxylated bisphenol- A dimethacrylate [Bis-EMA]; urethane dimethacrylate [UDMA]; triethylene glycol dimethacrylate [TEGDMA]) and different filler content (microhybrid and nanofilled). According to Distler and Kröncke , lactic acid and acetic acid account for 70% of the acids present in dental biofilm.13 Thus, we used these two acids in our study. The pH of acids used in our study was adjusted at 4, because previous studies have reported the pH of 4 as the lowest pH of dental plaque.13 Moreover, all specimens were stored in screw-top dark vials in an incubator at 37℃ during the study period in order to simulate the oral environment as much as possible. Surface resistance of materials to chemical degradation and their mechanical properties relate to wear resistance, and hardness measurement relatively determines this characteristic.212223
In our study, the microhardness of all groups decreased after 7 days of immersion compared to the baseline value. Longer storage time affects the filler surface or the fillermatrix bond.24 It has been confirmed that water and weak acids can cause inorganic filler surface degradation; this can be clearly seen in SEM images of specimens 7 days after immersion in distilled water and acidic solutions.25 Degradation of inorganic fillers may play an important role in microhardness reduction.26 This finding is in accord with the results of Honorio et al., and in contrast to those of Wan Bakar and Hashemi et al.172728
In our study, the microhardness of P90 silorane-based composite at all time points was lower than that of the two methacrylate-based composites. This difference in microhardness can be due to the filler type and content. P90 is a silorane-based microhybrid composite filled with fine quartz particles, whereas Z250 and Z350 contain zirconia-silica particles. The Knoop hardness is 820 for quartz and 1,160 for zirconia particles.29 This may be responsible for the lower hardness of P90. On the other hand, hardness is correlated with the degree of conversion (DC) and it has been shown that DC of silorane-based composites is lower than that of methacrylate-based resins explaining the lower baseline hardness of P90.3031 In our study, the microhardness of this composite significantly decreased after immersion, which is in contrast to the results of Kusgoz et al.31 They demonstrated that the microhardness of this composite remained unchanged after 7 and 30 days of water storage. Acids can release unreacted monomers in composites (due to low DC) via penetration into resin matrix and this issue may be responsible for the reduction in microhardness of P90 in our study.132
The microhardness of methacrylate-based composites also significantly decreased after 7 days of immersion but this reduction in Z350 was greater than the reduction in Z250. Z350 is a nanofilled composite. Its filler system is comprised of a combination of 20 nm silica nanofillers and 0.4 - 0.6 µm zirconia-silica nanoclusters.33 Although some studies have shown that this composite has mechanical properties similar to those of hybrid and midi-filled composites, its high surface/volume ratio due to the presence of silica particles may increase its water sorption and lead to the degradation of polymer-filler interface and possible drop in mechanical properties.910113435 On the other hand, Z350 contains large volumes of silane (γ-methacryl oxypropyltrimethoxysilane) due to high filler content and thus, may be more susceptible to hydrolysis and increased solubility. SEM image of this composite after immersion confirms this finding.
Lactic acid caused a greater reduction in microhardness than other solutions. Lactic acid is a carboxylic acid with -COOH and -OH functional groups. There is a high possibility that these functional groups form hydrogen bonds with the polar side of methacrylate monomer present in the matrix of Z250 and Z350, namely -OH in Bis-GMA, -OH in TEGDMA and Bis-EMA and -NH in UDMA causing greater water sorption and subsequently higher matrix softening. The SEM image of Z350 also confirms this theory. However, the SEM image of Z250 indicates scraped off filler particles, which may be responsible for decreased microhardness.
P90 is expected to have less solubility due to the presence of siloxane molecule. However, its microhardness significantly decreased and degradation of inorganic fillers was evident on the SEM image. It appears that solutions used in our study decreased its microhardness by affecting the silane coupling agent or the filler particles. On the other hand, it has been stated that chemical softening occurs when the solubility parameter of the resin matrix of composites is similar to the solubility parameter of storage media.36 No definite information is available regarding the solubility parameter of silorane but the proximity of the solubility parameter of P90 to that of solutions used in this study may also be responsible for the significant reduction of P90 microhardness compared to other composites. The aim of our study was to evaluate the immediate effect of organic acids in dental biofilm on microhardness of composites. In order to evaluate their effect of degradation, we need to store the samples longer.
Acknowledgement
The authors express their thanks to Tehran University of Medical Sciences, International Campus for financial support of this research.
References
1. Erdemir U, Yildiz E, Eren MM, Ozel S. Surface hardness evaluation of different composite resin materials: influence of sports and energy drinks immersion after a short-term period. J Appl Oral Sci. 2013; 21:124–131. PMID: 23739850.

2. Bagis YH, Baltacioglu IH, Kahyaogullari S. Comparing microleakage and the layering methods of siloranebased resin composite in wide Class II MOD cavities. Oper Dent. 2009; 34:578–585. PMID: 19830973.

3. Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005; 21:68–74. PMID: 15681004.

4. Hengtrakool C, Kukiattrakoon B, Kedjarune-Leggat U. Effect of naturally acidic agents on microhardness and surface micromorphology of restorative materials. Eur J Dent. 2011; 5:89–100. PMID: 21311608.

5. de Moraes RR, Marimon JL, Schneider LF, Sinhoreti MA, Correr-Sobrinho L, Bueno M. Effects of 6 months of aging in water on hardness and surface roughness of two microhybrid dental composites. J Prosthodont. 2008; 17:323–326. PMID: 18266654.

6. Yap AU, Tan SH, Wee SS, Lee CW, Lim EL, Zeng KY. Chemical degradation of composite restoratives. J Oral Rehabil. 2001; 28:1015–1021. PMID: 11722717.

7. Hannig C, Duong S, Becker K, Brunner E, Kahler E, Attin T. Effect of bleaching on subsurface micro-hardness of composite and a polyacid modified composite. Dent Mater. 2007; 23:198–203. PMID: 16546248.

8. Yap AU, Chew CL, Ong LF, Teoh SH. Environmental damage and occlusal contact area wear of composite restoratives. J Oral Rehabil. 2002; 29:87–97. PMID: 11844037.

9. Kalachandra S, Wilson TW. Water sorption and mechanical properties of light-cured proprietary composite tooth restorative materials. Biomaterials. 1992; 13:105–109. PMID: 1550893.

10. Curtis AR, Shortall AC, Marquis PM, Palin WM. Water uptake and strength characteristics of a nanofilled resin-based composite. J Dent. 2008; 36:186–193. PMID: 18237839.

11. da Silva EM, Gonçalves L, Guimarães JG, Poskus LT, Fellows CE. The diffusion kinetics of a nanofilled and a midifilled resin composite immersed in distilled water, artificial saliva, and lactic acid. Clin Oral Investig. 2011; 15:393–401.

12. Badra VV, Faraoni JJ, Ramos RP, Palma-Dibb RG. Influence of different beverages on the microhardness and surface roughness of resin composites. Oper Dent. 2005; 30:213–219. PMID: 15853107.
13. Distler W, Kröncke A. The acid pattern in human dental plaque. J Dent Res. 1983; 62:87–91. PMID: 6571872.

14. Borgström MK, Edwardsson S, Sullivan A, Svensäter G. Dental plaque mass and acid production activity of the microbiota on teeth. Eur J Oral Sci. 2000; 108:412–417. PMID: 11037757.

15. Namiot DB, Leszczyńska K, Namiot Z, Chilewicz M, Bucki R, Kemona A. The occurrence of Helicobacter pylori antigens in dental plaque; an association with oral health status and oral hygiene practices. Adv Med Sci. 2010; 55:167–171. PMID: 20934966.

16. Silva EM, Almeida GS, Poskus LT, Guimarães JG. Influence of organic acids present in the oral biofilm on the microtensile bond strength of adhesive systems to human dentin. J Biomed Mater Res B Appl Biomater. 2012; 100:735–741. PMID: 22190388.

17. Honório HM, Rios D, Francisconi LF, Magalhães AC, Machado MA, Buzalaf MA. Effect of prolonged erosive pH cycling on different restorative materials. J Oral Rehabil. 2008; 35:947–953. PMID: 18976266.
18. Asmussen E. Softening of BISGMA-based polymers by ethanol and by organic acids of plaque. Scand J Dent Res. 1984; 92:257–261. PMID: 6235572.

19. Eick JD, Smith RE, Pinzino CS, Kostoryz EL. Stability of silorane dental monomers in aqueous systems. J Dent. 2006; 34:405–410. PMID: 16288948.

20. Mujdeci A, Gokay O. Effect of bleaching agents on the microhardness of tooth-colored restorative materials. J Prosthet Dent. 2006; 95:286–289. PMID: 16616125.

21. Stockton LW, Williams PT, Attallah C. The effect of prolonged packing on the surface hardness of posterior composites. Oper Dent. 2002; 27:266–270. PMID: 12022458.
22. Wakamatsu Y, Kakuta K, Ogura H. Wear test combining simulated occlusal wear and toothbrush wear. Dent Mater J. 2003; 22:383–396. PMID: 14621003.
23. García-Godoy F, García-Godoy A, García-Godoy F. Effect of APF Minute-Foam on the surface roughness, hardness, and micromorphology of high-viscosity glass ionomers. J Dent Child (Chic). 2003; 70:19–23. PMID: 12762603.
24. Söderholm KJ, Zigan M, Ragan M, Fischlschweiger W, Bergman M. Hydrolytic degradation of dental composites. J Dent Res. 1984; 63:1248–1254. PMID: 6592209.

25. McKinney JE, Wu W. Chemical softening and wear of dental composites. J Dent Res. 1985; 64:1326–1331. PMID: 2936780.

26. Yap AU, Low JS, Ong LF. Effect of food-simulating liquids on surface characteristics of composite and polyacid-modified composite restoratives. Oper Dent. 2000; 25:170–176. PMID: 11203812.
27. Wan Bakar W, McIntyre J. Susceptibility of selected tooth-coloured dental materials to damage by common erosive acids. Aust Dent J. 2008; 53:226–234. PMID: 18782366.

28. Kamangar SS, Kiakojoori K, Mirzaii M, Fard MJ. Effects of 15% Carbamide peroxide and 40% hydrogen peroxide on the microhardness and color change of composite resins. J Dent (Tehran). 2014; 11:196–209. PMID: 24910696.
29. D'Alpino PH, Bechtold J, dos Santos PJ, Alonso RC, Di Hipólito V, Silikas N, Rodrigues FP. Methacrylate- and silorane-based composite restorations: hardness, depth of cure and interfacial gap formation as a function of the energy dose. Dent Mater. 2011; 27:1162–1169. PMID: 21925724.
30. Torres SA, Silva GC, Maria DA, Campos WR, Magalhães CS, Moreira AN. Degree of conversion and hardness of a silorane-based composite resin: effect of light-curing unit and depth. Oper Dent. 2014; 39:E137–E146. PMID: 24304340.

31. Kusgoz A, Ülker M, Yesilyurt C, Yoldas OH, Ozil M, Tanriver M. Silorane-based composite: depth of cure, surface hardness, degree of conversion, and cervical microleakage in Class II cavities. J Esthet Restor Dent. 2011; 23:324–335. PMID: 21977956.

32. Yesilyurt C, Yoldas O, Altintas SH, Kusgoz A. Effects of food-simulating liquids on the mechanical properties of a silorane-based dental composite. Dent Mater J. 2009; 28:362–367. PMID: 19662736.

33. Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003; 134:1382–1390. PMID: 14620019.

34. Beun S, Glorieux T, Devaux J, Vreven J, Leloup G. Characterization of nanofilled compared to universal and microfilled composites. Dent Mater. 2007; 23:51–59. PMID: 16423384.

35. Rodrigues Junior SA, Zanchi CH, Carvalho RV, Demarco FF. Flexural strength and modulus of elasticity of different types of resin-based composites. Braz Oral Res. 2007; 21:16–21. PMID: 17384850.

36. Sharafeddin F, Jamalipour G. Effects of 35% carbamide peroxide gel on surface roughness and hardness of composite resins. J Dent (Tehran). 2010; 7:6–12. PMID: 21998769.
Figure 1
The SEM image of (a) Z250, before immersion; (b) Z250, after 7 days immersion in distilled water; (c) Z250, after 7 days immersion in lactic; (d) Z250, after 7 days immersion in acetic acid (arrow, pitted area).

Figure 2
The SEM image of (a) Z350, before immersion; (b) Z350, after 7 days immersion in distilled water; (c) Z350, after 7 days immersion in lactic acid; (d) Z350, after 7 days immersion in acetic acid (arrow, eroded area).

Figure 3
The SEM image of (a) P90, before immersion; (b) P90, after 7 days immersion in distilled water; (c) P90, after 7 days immersion in lactic acid; (d) P90, after 7days immersion in acetic acid (arrow, pitted area).

Table 1
Materials and their composition used in the study
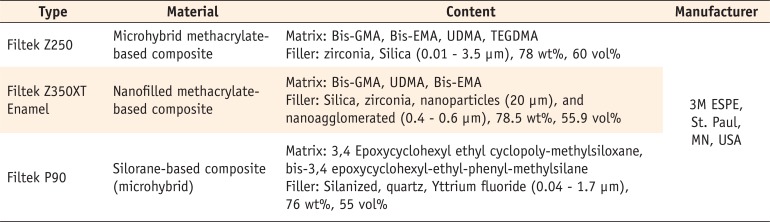
Table 2
Microhardness values (mean ± SD) of 3 composites vs. storage media and immersion time




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download