Abstract
Objectives
This study evaluated the solubility, dimensional alteration, pH, electrical conductivity, and radiopacity of root perforation sealer materials.
Materials and Methods
For the pH test, the samples were immersed in distilled water for different periods of time. Then, the samples were retained in plastic recipients, and the electrical conductivity of the solution was measured. The solubility, dimensional alteration, and radiopacity properties were evaluated according to Specification No. 57 of the American National Standards Institute/American Dental Association (ANSI/ADA). Statistical analyses were carried out using analysis of variance (ANOVA) and Tukey's test at a significance level of 5%. When the sample distribution was not normal, a nonparametric ANOVA was performed with a Kruskal-Wallis test (α = 0.05).
Results
The results showed that white structural Portland cement (PC) had the highest solubility, while mineral trioxide aggregate (MTA)-based cements, ProRoot MTA (Dentsply-Tulsa Dental) and MTA BIO (Ângelus Ind. Prod.), had the lowest values. MTA BIO showed the lowest dimensional alteration values and white PC presented the highest values. No differences among the tested materials were observed in the the pH and electrical conductivity analyses. Only the MTA-based cements met the ANSI/ADA recommendations regarding radiopacity, overcoming the three steps of the aluminum step wedge.
Conclusions
On the basis of these results, we concluded that the values of solubility and dimensional alteration of the materials were in accordance with the ANSI/ADA specifications. PCs did not fulfill the ANSI/ADA requirements regarding radiopacity. No differences were observed among the materials with respect to the pH and electrical conductivity analyses.
Root canal perforation is an artificial communication between the pulp cavity and the periodontal tissues.1 Many materials have been used in the treatment of endodontic perforations, but different results show that there is no ideal material.2 In 1993, a mineral trioxide aggregate (MTA) was used to seal lateral root perforations and as a root-end filling material.3 Additional dental applications of MTA have been subsequently proposed, including direct pulp capping, external root resorption repair, and its use in the apexification therapy.3,4 This material has excellent physical, chemical, and biological properties.4,5,6,7,8,9,10,11,12,13 However, MTA presents one of the longest setting time among the root canal perforation sealer materials, forming a colloidal gel that solidifies in 2 hours and 30 minutes.14,15 In general, it is desirable for a material to harden as soon as possible in order to avoid significant contractions. Other disadvantages of the MTA are its high cost, its poor adhesion to dentin, and its low resistance to compression.3,5,16
MTA cements are derived from ordinary type I Portland cement (PC) with 4 : 1 proportion of bismuth oxide added for radiopacity.11,14,17 PC, a material used in civil engineering, is the main component of the MTA, and similarities in the physicochemical and biological properties of both materials have been demonstrated.4,5,14,17,18 Thus, PC has considerable potential for use as a dental material. In Brazil, there are many different types of PC, classified according to their compositions.19 Type II, type V, and white PC are examples of PC with additives.20,21 Silva Neto et al. clinically, radiologically, and histologically evaluated root perforations treated with MTA or PC with additives and observed that all tested materials induced new-bone formation.12
Newly proposed root perforation sealing materials should have their physicochemical properties tested. Even though type I PC is the major constituent of MTA, important differences can be noted in relation to the material's mass characteristics and physicochemical properties.5,7,21,22 Based on this and the perspective to develop new materials, the aim of this study was to evaluate the solubility, dimensional change, pH, electrical conductivity, and radiopacity of white structural and type V gray PC, MTA BIO, and ProRoot MTA.
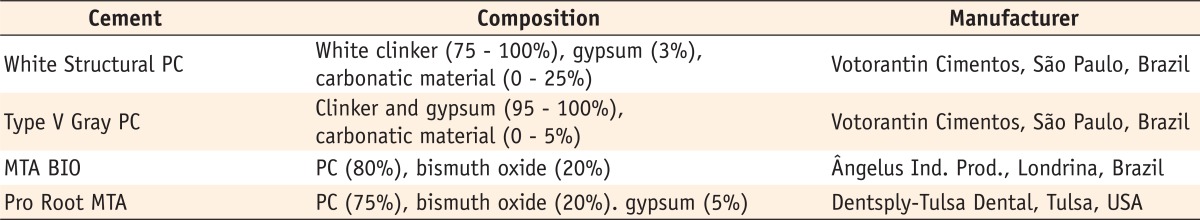
The materials evaluated in the present study and their chemical compositions, according to the manufacturers, are described in Table 1.
The solubility, dimensional change, and radiopacity were determined in accordance with methods recommended by the American National Standards Institute/American Dental Association (ANSI/ADA) specification number 57 for endodontic sealing materials and as suggested by Carvalho-Junior et al.23,24 The MTA-based cements were manipulated according to the manufacturer's instructions at a water-to-powder ratio of 1 : 3. The PC samples were prepared using the same water-to-powder ratio as that of MTA.
Five samples (thickness, 1.5 mm; inner diameter, 7.75 mm) of each material were used. The tested materials were prepared and inserted into the molds. In sequence, 0.5-mm diameter waterproof nylon was inserted in the softened cements. After three times the setting time, the samples were removed from the molds and weighed on a precision scale of 0.0001 g (Ohaus Corporation, Parsippany, NJ, USA). The samples suspended by the nylon were placed in wide-mouthed plastic recipients containing 7.5 mL of distilled water and maintained hermetically closed in an incubator at a constant temperature of 37 ± 2℃ for 24 hours. After this time, the samples were removed; the excess water was removed with absorbent paper. The samples were maintained in a dehumidifier for 24 hours, after which they were weighed a second time. Each material's solubility was considered to be the percentage loss of its mass with respect to the initial mass. Five repetitions were considered for each material.
Five Teflon (polytetrafluroethylene, Habia Teknofluor AB, Knivsta, Sweden) molds were prepared; they had a height of 3.58 mm and diameter of 3.0 mm. The wells of these molds were filled with the material to be tested, stored, and had their ends ground with a wet 600-grit SiC paper to obtain a regular surface. The samples were removed from the mold, their length was measured, and they were stored in a vessel containing 2.24 mL of distilled water at 37℃ for 30 days. The samples were removed from the containers, dried, and measured again for length. The percentage of the dimensional alterations was calculated by using the following formula: [(L30 - L) / L] × 100, where L30 denotes length of the sample after 30 days and L represents the initial length of the sample. The arithmetic mean of 5 replicates for each sealer was recorded as the dimensional alteration of the cement tested.
Five samples (thickness: 1.5 mm; inner diameter: 7.75 mm) were used for each material. Each cylinder was sealed in a flask containing 7.5 mL of distilled water. Distilled water pH measurements were taken with a pH meter (Corning Inc., Corning, NY, USA) at 1, 3, 5, 15, and 30 minutes; 1, 2, 3, 4, 6, 9, 12, 24, 48, and 72 hours; 4, 6, 7, 15, and 30 days after spatulation. During the experiment, pH was analyzed for each sample in the same plastic recipient without liquid substitution. It was measured five times for each material. Mean values and standard deviations were recorded for all measurements.
After the pH analysis, the samples were retained in the plastic recipients, and the electrical conductivity of the solutions was measured. All 5 samples of each material were analyzed with a conductivimeter (Marconi Equip. Ltda, Piracicaba, SP, Brazil). This device was calibrated according to a calibration curve obtained from a 1.412-µS/cm solution.
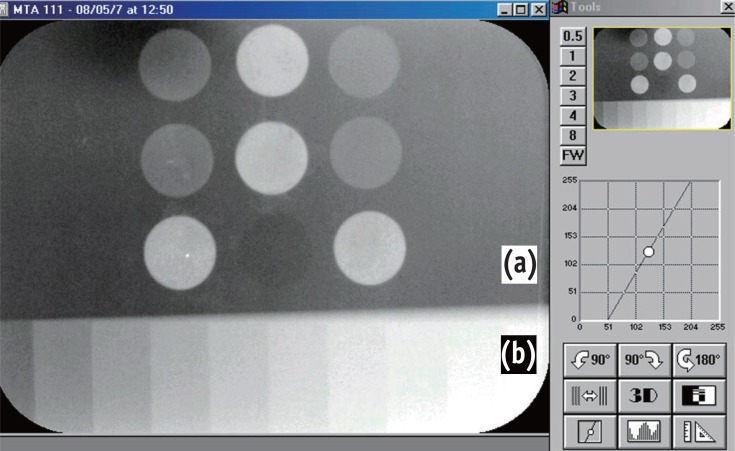
Five acrylic plates (22 × 45 × 1 mm) with 6 holes with a depth of 1 mm and an internal diameter of 5 mm were fulfilled with the tested cements. For the radiographic exposure, each acrylic plate containing the cements was positioned together with another acrylic plate (13 × 45 × 1 mm), which contained a graduated aluminum step wedge varying from 1 to 10 mm in thickness in uniform steps of 1 mm. The set of plates corresponds exactly to the sensor size from the Digora system (Soredex Orion Corporation, Helsinki, Finland), which was used for the data collection. A 70-kVp, 8-mA radiograph machine Spectro 70X (Dabi Atlante Inds. Médico Odontologicas Ltda, Ribeirão Preto, Brazil) was used. The focus-object distance was 30 cm, and the exposure time was 0.2 seconds. The sensor, after being exposed, was inserted into the laser optical reader of Digora for Windows 5.1 software. The same phosphor plate was used for all exposures. The system performed a radiographic density reading over images of each cement sample revealed on screen, and a reading of steps on an aluminum step wedge, resulting in a numeric value for each reading. After evaluating the set of 5 acrylic plates, 5 measurements for each type of cement and for each step of the aluminum scale were obtained. Mean values were taken by a single evaluator previously trained and blinded with respect to the different groups.
Statistical analyses were carried out for solubility, pH, electrical conductivity, and radiopacity using analysis of variance (ANOVA) and Tukey's test at a significance level of 5%. When the sample distribution was non-normal, a nonparametric ANOVA was performed using a Kruskal-Wallis test (α = 0.05). The tests were performed with the SPSS for Windows statistical software version 21 (SPSS Inc., Chicago, IL, USA).
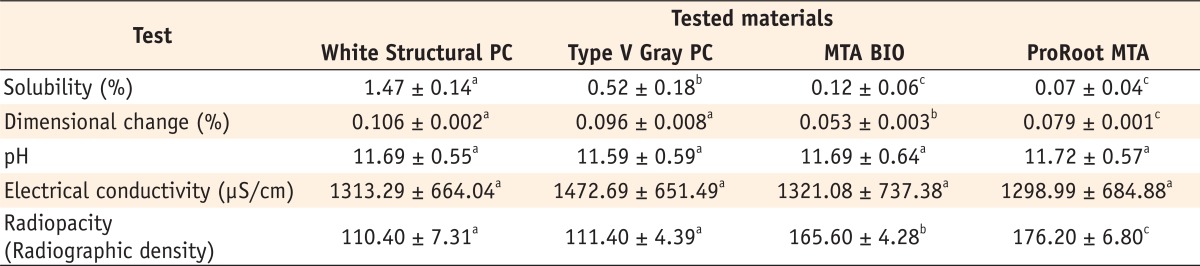
Table 2 presents the mean values and standard deviations of the physiochemical properties of the tested materials.
White structural PC had the highest mean value for solubility (p < 0.05, Table 2), while ProRoot MTA had the lowest (p < 0.05). Type V PC presented intermediary values, different from those of the other materials (p < 0.05). There were statistically significant differences between MTA-based and PC materials (p < 0.05). No difference was observed between ProRoot MTA and MTA BIO (p > 0.05).
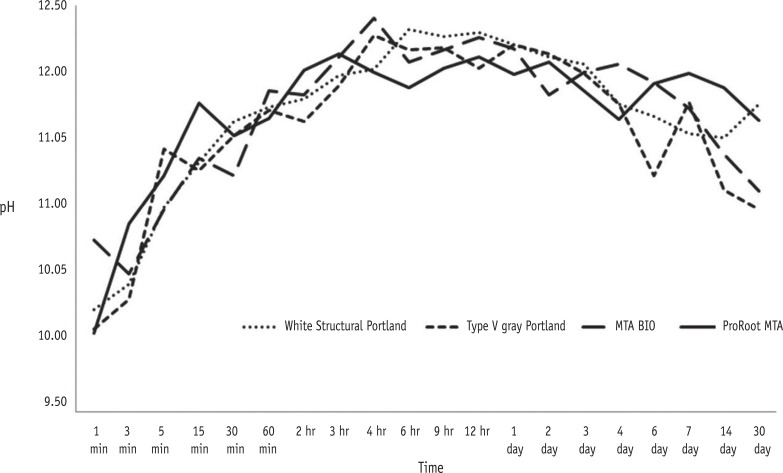
The change in pH as a function of time is shown in Figure 1. The pH values for the cements ranged from 9.84 to 12.29. At 1-minute immersion, significant differences were observed from the values of the other time periods (p < 0.05). Alternatively, similar pH values were observed at other spatulation times (p > 0.05). No significant difference was observed in the mean values for the pH reading of each tested material (p > 0.05, Table 2).
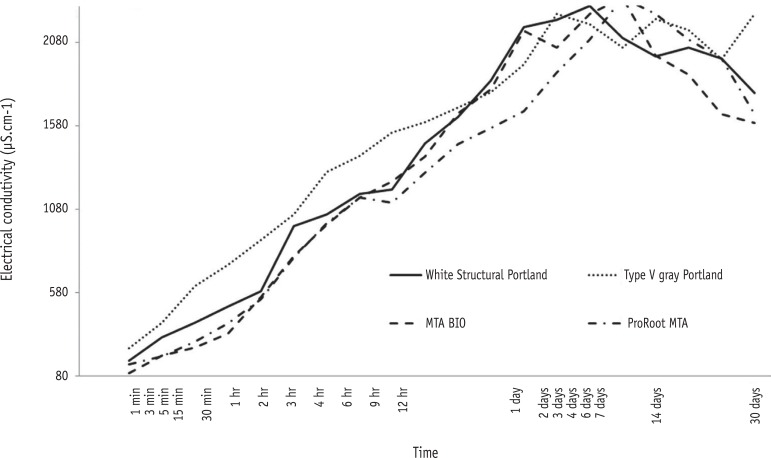
The results indicated that the conductivity of the materials was not statistically different (p > 0.05). At 1 minute, a significant difference in conductivity was observed (p < 0.05). Alternatively, at other periods of time, differences were not observed between samples (p > 0.05), but these results were different from those obtained at 1 minute (p < 0.05, Figure 2).
The radiopacity of the materials was compared using the aluminum step wedge, as illustrated in Figure 3. MTA-based cements presented the highest radiopacity mean values among the tested materials, overcoming the three steps of the aluminum step wedge (132.47), which is the minimum recommended by the ANSI/ADA, while PC did not meet this requirement.23 The statistical analysis demonstrated the difference among the tested materials (p < 0.05, Table 2).
An ideal root perforation sealing material should be dimensionally stable, radiopaque, easy to be manipulated, atoxic, noncarcinogenic, nongenotoxic, and biocompatible, and if possible, it should stimulate the healing process.1,6,8,9,10,11 Furthermore, this material should present adequate solubility in oral fluids, satisfactory working time, and antimicrobial activity.3,4,5,7,17 Numerous studies have been conducted to study the similarities in the physicochemical properties of MTA and ordinary type I PC.6,14,25,26,27 In the present study, the solubility, pH, electrical conductivity, and radiopacity of white structural and type V gray PC were analyzed and compared to those of MTA-based cements with the aim of finding a new root perforation sealing material that is as equally effective as MTA.
Due to a lack of specific standards to test the physical properties of root perforation sealing materials, published studies have followed the ANSI/ADA specification number 57 for endodontic sealing materials to support and to reference studies on the analysis of the physicochemical properties of MTA and PC.28,29 Under clinical conditions, both root filling and perforation sealing materials remain in close contact with the periodontal tissues.30,31 Thus, the ANSI/ADA standard is assumed to be applicable to the materials under investigation following the modifications proposed by Carvalho-Junior et al., allowing the reduction of 80% in volume of the materials for conducting tests, without any involvement or interference in the results.23,24
MTA is a powder that consists of fine hydrophilic particles that harden when they come in contact with water.3,6,7,11,16 The physicochemical characteristics of MTA are influenced by several factors such as the type of MTA, type of storage media, type of vehicle, size of the particles, temperature and humidity at the application, amount of air trapped in the mixture, the mixing procedure itself, and the powder-to-water ratio.26,28,32 Fridland and Rosado noted that the standardization of methods to study the physicochemical properties of MTA is hampered by the lack of control of several of the above-mentioned factors; thus, different results might be obtained.30
ProRoot MTA and MTA BIO patents describe these materials as PC type I, with gypsum addition for setting time control and bismuth oxide for radiopacity improvement.17,27 PC can be grouped into two main categories: ordinary (type I) and with additives.20 Type I PC is composed of clinker and gypsum. Type II, the most common type of PC, contains granulated slag from a blast furnace (6 - 34%), pozzolanic material (6 - 14%), and carbonatic material and clinker (56 - 94%). The main elements of type IV PC are pozzolanic (15 - 50%) and carbonatic (0 - 5%) materials. The pozzolanic material gives the cement reduced permeability, increased ionic diffusibility, increased stability and durability, improved performance as compared to the action of sulfates/alkali-aggregate reaction, reduced hydration heat, and increased compressive strength of the cement. Song et al. evaluated the cytotoxicity of a pozzolan cement (Endocem, Maruchi, Wonju, Korea) and other root-end filling materials by using human periodontal ligament cells and observed that the cytotoxicity of endocem was comparable to that of ProRoot MTA and MTA Angelus.10 Type V PC has different specifications of limestone and clay. It receives a special grilling treatment during the production of the clinker, offering high resistance to the material in a shorter period of time, when compared to other cements.19 Silva Neto et al. evaluated the use of MTA and PC with additives (white, type II, and type V) as furcation perforation repair materials and assessed their biocompatibility. For the authors, MTA and PC with additives have similar biocompatibility.13 Despite the already demonstrated biocompatibility, information regarding the physicochemical properties of PC with additives is scant.
In general, root perforation sealing materials should present low solubility when in contact with the tissue fluid. Solubility shows a significant trend that follows the amount of water used when preparing the mixture.11,16,30 In this study, MTA-based cements were prepared following the manufacturers' recommendations. PC samples were prepared using the same method, since a specific ratio for the use of PC in dentistry has not been determined. All tested materials meet the ANSI/ADA specification, according to which a root canal sealer should not present solubility higher than 3%.23 It was found that both MTA-based cements were less soluble than the PC, which is consistent with the results of the other studies.3,5,26,30,33 However, Fridland and Rosado observed increased solubility values in a long-term study.34 The difference in solubility between MTA and PC is probably related to the differences in the chemical surface composition of the cements after the setting reaction.32 MTA-based cements present less sulfur and potassium but have increased calcium content at their surface, while PC presents a higher sulfur content, which is related to the greater amount of gypsum.22 Moreover, the addition of bismuth oxide to MTA, which is insoluble in water, is the cause of its insolubility.30 The solubility of white structural PC is significantly greater than that of type V gray PC. The solubility of this cement is related to the lower content of clinker in its constitution, which results in a less resistant structure.19 Lower setting time may be one of the reasons for the greater solubility of PC.32 Bortoluzzi et al. observed that the addition of CaCl2 to white PC reduced its solubility.33 It is important to note that the solubility testing standards recommend the immersion of materials only after the setting is completed; it is impossible to achieve this under clinical conditions since the materials are immediately in contact with oral fluids.29,30,33
Dimensional change is another important property that needs to be considered, since a change in dimensions, possibly leading to contraction, would have a negative impact on the material's ability to seal a perforation site.7,12,26,35 Considering the dimensional change test, the ANSI/ADA standardization states that the mean linear shrinkage of the sealer shall not exceed 1% or 0.1% in the case of expansion.23 In the present study, all the materials tested showed expansion on setting and these values were in accordance with the ANSI/ADA specification. The slight expansion on the setting observed may be explained by the water absorption by the cements.7,16 This fact is helpful in ensuring that the seal is present upon setting and consequently, reducing subsequent leakage without increasing the risk of the development of cracks or root fracture.26,35
All materials evaluated in this study promoted an alkaline pH, with values ranging from 9.84 to 12.29, which remained high until the end of the experiment. This can be explained by the hydration reaction, which is primarily a hydrolysis of silicates, producing a hydrate of lower basicity calcium silicate (CHS gel). This releases lime and separates into calcium hydroxide, along with calcium silicates forming CHS gel and calcium hydroxide.7,16,34 Duarte et al. observed low pH values, which could be related to the different methodology used in the experiment.36 MTA and PC-based cements are rich in calcium ions,14,37,18,26, which are converted to calcium hydroxide, upon contact with water, and dissociate into calcium and hydroxyl ions, increasing the pH of the solution.36 An immediate increase in pH after material immersion is due to the reaction that takes place when the cement comes into contact with water, resulting in a saturated calcium hydroxide solution.26 The values for the release of calcium and hydroxide ions are higher during the initial period, after which they tend to decrease.36,38 Thus, the variation in the concentration of calcium and hydroxyl ions leads to different pH values (Figure 1). MTA and PC consist mainly of calcium; however, a significant difference in the calcium concentration between gray and white MTA (42.37 wt% and 45.09 wt%, respectively) and PC (51,82 wt%) is observed.37 Gonçalves et al. observed that MTA BIO released significantly more calcium ions into solution than ProRoot MTA. This was attributed to the setting time of the material as well as the original concentration of calcium.39
Electrical conductivity of the material is related to the quantity of ions released into the medium and is directly proportional to material solubility.36,38 During the sample's solubilization process, the components that were the most soluble in water were the first to release ions into the solution. It has been reported that MTA- and PC-based cements behave in the same manner, releasing a considerable amount of ions in the beginning, with a tendency to release fewer ions with an increase in the evaluation period.38 This fact could explain the decrease in electrical conductivity values observed after 7 days (Figure 2). Considering the complexity of the materials, we found that the ionic equilibrium is equally complex. Calcium is the main element present in these cements and should be considered to have the common-ion effect. The conductivity values of the cements were similar, leading to the conclusion that all samples were affected in the same way by the solvent and that it was possible to observe stabilization as a consequence of the solution saturation.36 Moreover, the volume of solvent used in the test was insufficient (7.5 mL). In the present study, the solution was not removed or exchanged once the samples were immersed; thus, the results obtained were different from those observed by Santos et al.38
Root canal sealer should present higher radiopacity values than 3-mm thick Al and be easily distinguishable from dentin and gutta-percha on radiographs.3,5,21,23 The same should be expected of root perforation sealing materials. According to the present results, ProRoot MTA was the most radiopaque material followed by MTA BIO. This is explained to be a consequence of the lower concentration of the bismuth oxide in its composition, thus corroborating with the present study's findings.26,32 MTA-based cements contain approximately 13.63 - 16.9 wt.% of bismuth.37 The radiopacity values recorded in the present study are in accordance with the results of previous studies.21,32 White structural and type V gray PC presented the lowest radiopacity and do not have sufficient radiopacity to be visualized radiographically; thus, radiopacifier agents have been added to this cement.40 Despite the favorable results, the effect of adding new materials to MTA and PC should be further investigated because some of these properties may be improved and others may be even lost.28
On the basis of the results of this study, it seems that all tested materials presented values of solubility and dimensional change in accordance with the ANSI/ADA specification. No difference in pH was observed, and it was maintained in the alkaline range over the entire test period. All cements presented similar electrical conductivity. Only the MTA-based cements met the ANSI/ADA recommendations referring to radiopacity.
References
1. Krupp C, Bargholz C, Brüsehaber M, Hülsmann M. Treatment outcome after repair of root perforations with mineral trioxide aggregate: a retrospective evaluation of 90 teeth. J Endod. 2013; 39:1364–1368. PMID: 24139255.
2. Park DS, Sohn SJ, Oh TS, Yoo HM, Park CJ, Yim SH, Lee YK, Kye SB. An electrochemical study of the sealing ability of three retrofilling materials. J Korean Acad Conserv Dent. 2004; 29:365–369.

3. Darvell BW, Wu RC. "MTA"-an Hydraulic Silicate Cement: review update and setting reaction. Dent Mater. 2011; 27:407–422. PMID: 21353694.

4. Malhotra N, Agarwal A, Mala K. Mineral trioxide aggregate: a review of physical properties. Compend Contin Educ Dent. 2013; 34:e25–e32. PMID: 23627406.
5. Borges AH, Pedro FL, Miranda CE, Semenoff-Segundo A, Pécora JD, Filho AM. Comparative study of physicochemical properties of MTA-based and Portland cements. Acta Odontol Latinoam. 2010; 23:175–181. PMID: 21638956.
6. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413. PMID: 20171353.

7. Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007; 40:462–470. PMID: 17459120.

8. Lee JH, Shon WJ, Lee W, Baek SH. The effect of several root-end filling materials on MG63 osteoblast-like cells. J Korean Acad Conserv Dent. 2010; 35:222–228.

9. Saxena P, Gupta SK, Newaskar V. Biocompatibility of root-end filling materials: recent update. Restor Dent Endod. 2013; 38:119–127. PMID: 24010077.

10. Song M, Yoon TS, Kim SY, Kim E. Cytotoxicity of newly developed pozzolan cement and other root-end filling materials on human periodontal ligament cell. Restor Dent Endod. 2014; 39:39–44. PMID: 24516828.

11. Cavenago BC, Pereira TC, Duarte MA, Ordinola-Zapata R, Marciano MA, Bramante CM, Bernardineli N. Influence of powder-to-water ratio on radiopacity, setting time, pH, calcium ion release and a micro-CT volumetric solubility of white mineral trioxide aggregate. Int Endod J. 2014; 47:120–126. PMID: 23647286.

12. Silva Neto JD, Brito RH, Schnaider TB, Gragnani A, Engelman M, Ferreira LM. Root perforations treatment using mineral trioxide aggregate and Portland cements. Acta Cir Bras. 2010; 25:479–484. PMID: 21120277.

13. Silva Neto JD, Schnaider TB, Gragnani A, Paiva AP, Novo NF, Ferreira LM. Portland cement with additives in the repair of furcation perforations in dogs. Acta Cir Bras. 2012; 27:809–814. PMID: 23117614.

14. Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005; 38:834–842. PMID: 16218977.

15. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993; 19:591–595. PMID: 8151252.

16. Chang SW. Chemical characteristics of mineral trioxide aggregate and its hydration reaction. Restor Dent Endod. 2012; 37:188–193. PMID: 23429542.

17. Estrela C, Bammann LL, Estrela CR, Silva RS, Pécora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J. 2000; 11:3–9. PMID: 11210272.
18. Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: a review of the constituents and biological properties of the material. Int Endod J. 2006; 39:747–754. PMID: 16948659.

19. ABCP. Basic guide of Portland cement utilization. 7th ed. São Paulo: Brazilian Association of Portland Cement;2002. p. 28.
20. Garboczi EJ, Bullard JW. Shape analysis of a reference cement. Cem Concr Res. 2004; 34:1933–1937.

21. Borges AH, Pedro FL, Semanoff-Segundo A, Miranda CE, Pécora JD, Cruz Filho AM. Radiopacity evaluation of Portland and MTA-based cements by digital radiographic system. J Appl Oral Sci. 2011; 19:228–232. PMID: 21625738.

22. Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005; 21:731–738. PMID: 15935463.

23. ANSI/ADA. Specification No. 57. Endodontic sealing materials. Chicago: American Dental Association Publishing;2000.
24. Carvalho-Junior JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa-Neto MD. Solubility and dimensional change after setting of root canal sealers: a proposal for smaller dimensions of test samples. J Endod. 2007; 33:1110–1116. PMID: 17931945.
25. Tanomaru-Filho M, da Silva GF, Duarte MA, Gonçalves M, Tanomaru JM. Radiopacity evaluation of root-end filling materials by digitization of images. J Appl Oral Sci. 2008; 16:376–379. PMID: 19082394.

26. Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and portland cement. J Endod. 2006; 32:193–197. PMID: 16500224.

27. Hwang YC, Lee SH, Hwang IN, Kang IC, Kim MS, Kim SH, Son HH, Oh WM. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e96–e102. PMID: 19157923.

28. Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006; 32:569–572. PMID: 16728254.

29. Duarte MA, Alves de Aguiar K, Zeferino MA, Vivan RR, Ordinola-Zapata R, Tanomaru-Filho M, Weckwerth PH, Kuga MC. Evaluation of the propylene glycol association on some physical and chemical properties of mineral trioxide aggregate. Int Endod J. 2012; 45:565–570. PMID: 22720325.

30. Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod. 2003; 29:814–817. PMID: 14686812.

31. de Martins GR, Carvalho CA, Valera MC, de Oliveira LD, Buso L, Carvalho AS. Sealing ability of castor oil polymer as a root-end filling material. J Appl Oral Sci. 2009; 17:220–223. PMID: 19466255.
32. Danesh G, Dammaschke T, Gerth HU, Zandbiglari T, Schäfer E. A comparative study of selected properties of ProRoot mineral trioxide aggregate and two Portland cements. Int Endod J. 2006; 39:213–219. PMID: 16507075.

33. Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009; 35:550–554. PMID: 19345803.

34. Fridland M, Rosado R. MTA solubility: a long term study. J Endod. 2005; 31:376–379. PMID: 15851933.

35. Wiltbank KB, Schwartz SA, Schindler WG. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and Portland cement. J Endod. 2007; 33:1235–1238. PMID: 17889697.

36. Duarte MA, Demarchi AC, Yamashita JC, Kuga MC, Fraga Sde C. pH and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95:345–347. PMID: 12627108.

37. Estrela C, Sousa-Neto MD, Guedes OA, Alencar AH, Duarte MA, Pécora JD. Characterization of calcium oxide in root perforation sealer materials. Braz Dent J. 2012; 23:539–546. PMID: 23306231.

38. Santos AD, Moraes JC, Araújo EB, Yukimitu K, Valério Filho WV. Physico-chemical properties of MTA and a novel experimental cement. Int Endod J. 2005; 38:443–447. PMID: 15946264.

39. Gonçalves JL, Viapiana R, Miranda CE, Borges AH, Cruz Filho AM. Evaluation of physico-chemical properties of Portland cements and MTA. Braz Oral Res. 2010; 24:277–283. PMID: 20877963.

40. Húngaro Duarte MA, de Oliveira El Kadre GD, Vivan RR, Guerreiro Tanomaru JM, Tanomaru Filho M, de Moraes IG. Radiopacity of portland cement associated with different radiopacifying agents. J Endod. 2009; 35:737–740. PMID: 19410095.

Figure 3
Illustration of the radiographic density reading over images by the DigoraTM system. (a) Acrylic plate (22 × 45 × 1 mm) with holes having a depth of 1 mm and an internal diameter of 5 mm filled with the tested cements; (b) Graduated aluminum step wedge varying from 1 to 10 mm in thickness in uniform steps of 1 mm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download