Abstract
Objectives
To comparatively evaluate the efficacy of photo-activated disinfection (PAD), calcium hydroxide (CH) and their combination on the treatment outcome of indirect pulp treatment (IPT).
Materials and Methods
Institutional ethical clearance and informed consent of the patients were taken. The study was also registered with clinical registry of India. Sixty permanent molars exhibiting deep occlusal carious lesion in patients with the age range of 18 - 22 yr were included. Clinical and radiographic evaluation and set inclusion and exclusion criteria's were followed. Gross caries excavation was accomplished. In group I (n = 20) PAD was applied for sixty seconds. In group II (n = 20), CH was applied to the remaining carious dentin, while in group III (n = 20), PAD application was followed by CH placement. The teeth were permanently restored. They were clinically and radiographically followed-up at 45 day, 6 mon and 12 mon. Relative density of the remaining affected dentin was measured by 'Radiovisiography (RVG) densitometric' analysis.
Results
Successful outcome with an increase in radiographic grey values were observed in all three groups. However, on inter-group comparison, this change was not significant (p > 0.05).
Conclusions
PAD and CH both have equal disinfection efficacy in the treatment of deep carious dentin. PAD alone is as effective for treatment of deep carious lesion as calcium hydroxide and hence can be used as an alternative to CH. They can be used independently in IPT, since combining both does not offer any additional therapeutic benefits.
Vital pulp therapy aims to preserve and maintain the integrity of the pulp that has been compromised by caries, trauma or restorative procedures.1 Indirect pulp treatment (IPT) is one such therapeutic modality and is based on the research of Fusayama et al. who demonstrated that in acute caries, discoloration (affected dentin) preceded the presence of microorganisms (infected dentin).2 The success of IPT depends on adequate reduction of the microbial load, reparative ability of the pulp-dentin complex and placement of a suitable coronal restoration.
Role of remineralizing materials in IPT have been widely investigated, but with conflicting conclusions. Calcium hydroxide (CH) has emerged as the "gold standard" with a success rate of 92 - 97%.3 On the contrary, Eidelman et al. and Pinto et al. have documented similar clinical, radiographic, microbiological and ultra-structural outcome of IPT performed with either CH or inert materials.4,5 This implies that the success of this technique is not dependent on the material applied per se, but on an effective antimicrobial treatment of the deep layers of carious dentin. Another pre-requisite for favorable outcome in IPT is an effective coronal seal. Adequate disinfection is accomplished by physical removal of the infected dentin and entombing the remaining microbes. This creates the conditions for caries arrest and remineralization. However, determining the extent of carious tissue excavation and achieving long-term seal by current restorative materials remain a pitfall. In this context, it is desirable to investigate an effective way of disinfecting the remaining carious dentin.
Photo-activated disinfection (PAD) is an antimicrobial aid that utilizes a photoactive compound (e.g. toluidine blue O, methylene blue) and a light of specific wavelength (600 - 750 nm) to produce oxygen-based free radicals that exterminate the bacteria. PAD is an integral part of the disinfection regimen for root canal therapy due to its dual selectivity, minimal chance of microbial resistance, broad spectrum, instant bacterial killing and ability to target biofilm bacteria. In vitro studies that documented the efficacy of PAD against cariogenic bacteria concluded that it could be an attractive proposition for caries management.6,7 The aim of the present study was to evaluate the role of PAD on the outcome of IPT. The hypothesis was that PAD and CH would result in comparable treatment outcome.
Ethical clearance was obtained from the Institute Research Ethics Committee (Ref. No. IESC/T-240/2010). Three study groups were defined on the basis of the material applied, i.e. PAD (Group I), CH (Group II) and PAD followed by CH application (Group III). Subjects between the age group of 18 - 22 years with occlusal carious lesion on molars were included in the study. A detailed clinical history regarding the type, duration, frequency, triggering stimuli and relief from pain was obtained to clinically establish the diagnosis of reversible pulpitis. Subjects with symptoms of irreversible pulpitis/apical periodontitis were excluded. Pre-treatment standardized intraoral periapical and bitewing radiographs were exposed on a size-2 CMOS RVG sensor (Kodak RVG 5100 digital radiography system, Eastman Kodak Company, Rochester, NY, USA) held in a sensor positioning device (RINN XCP-ORA, Dentsply Rinn, Elgin, IL, USA). For all the radiographs, a customized polyvinyl siloxane (Aquasil, Dentsply International Inc., York, PA, USA) bite block was fabricated to register and reproduce the same angulation at subsequent follow-ups. The X-ray unit (ENDO-ACP, Villa sistemi medicali, Milano, Italy) was operated at 70 kV and 7 mA with an exposure time set at 0.12 seconds.
A block randomization method was followed. Sixty teeth were allocated to the pre-defined study groups (n = 20) by an independent statistician through a computer generated random number table. Patient information sheet was provided. The subjects were blinded to the material applied. Written informed consent was obtained.
A single operator who was a post graduate student performed all the procedures for IPT on patients under direct supervision of two faculty members. The tooth was anaesthetized (Lox 2%, Lidocaine HCl Injection, Neon Laboratory Ltd., Mumbai, India) and isolated under a rubber dam (Hygenic Dental Dam, Coltène/Whaledent Inc., Cuyahoga Falls, OH, USA). Unsupported enamel and caries from the lesion walls were removed with a sterile #245 tungsten carbide bur (SS White burs Inc., Lakewood, NJ, USA) operated in a high speed air water spray handpiece (Extra Torque 606 C Turbine, KaVo Dental Excellence, Santa Catarina, Brazil). A round tipped probe aided in 'tactile detection' of dentin consistency. In the area deemed "at risk" for pulp exposure, the softened, humid, yellow or light-brown carious dentin that did not offer any resistance to probing was removed with a sterile #6 or #8 carbide bur (SS White burs Inc.) at a low speed handpiece (Ti-Max, NSK, Tochigi, Japan). The darker and harder dentin was preserved.
In group I, PAD solution for caries (0.01 mg/mL tolonium chloride, manufacturer, city, country) was applied with a brush for 60 seconds and irradiated for 60 seconds with PAD system (PAD, Denfotex Light Systems Ltd., Fife, UK). The output was set at 100 mW. The fibre-optic tip was kept at a distance of 2 mm from the carious dentin (Figure 1). The cavity was rinsed with distilled water (Shree Krishna Keshav Laboratory Ltd., Ahmedabad, India) and gently air dried. Cavit G (3M ESPE AG, Seefeld, Germany) was then applied as a sub-base. In group II, CH (Dycal, Dentsply Caulk, Dentsply International Inc., Milford, DE, USA) was applied to the remaining carious dentin without PAD treatment. In group III, PAD application was followed by CH application. The cavities in all three groups were restored with zinc-phosphate base (Mission Dental Inc., Charleston, SC, USA) and silver amalgam (Dispersalloy, Dentsply Int. Inc.; Triple distilled mercury, Chemident Srl, Grosseto, Italy) after application of 2 coats of varnish on the base and cavity walls.
The mid-treatment exclusion criteria included pulp exposure during caries removal and dislodged coronal restoration/tooth fracture. The cases were followed up clinically and radiographically at 45 days, 6 months and 12 months by an independent endodontist who was blinded to the treatment protocol. The criteria used for determining the successful outcome were absence of (1) spontaneous pain and/or sensitivity to percussion, (2) fistula/swelling, (3) pathological mobility, (4) widening of periodontal ligament space, (5) radiolucency at the furcal and/or periapical region, and (6) internal/external dentin resorption.
Radiographic evaluation also included measuring of relative density of the remaining dentin using a software available on RVG machine. The 'Intraoral' option in the main menu bar of the Kodak RVG Trophy Imaging Software 6.12.10.0 was used. The 'Tools window' was navigated and 'Densitometric analysis' was clicked. Two defined and repeatable points (i.e. A and B) were identified in the immediate post-treatment and subsequent follow-up bitewing radiographs (Figure 2). 'A' denoted a mark 0.5 mm below the deepest part of the restored cavity and 'B' referred to a mark exactly perpendicular to A on the roof of the pulp chamber. The average grey value along the line joining the two points was noted and correlated with the data obtained on subsequent follow-up. Statistical tests were performed using the Stata 11.0 software (Stata Corp, College Station, TX, USA). The p value was set at 0.05.
Two teeth had to be excluded due to mid-treatment tooth/restoration fracture. A recall rate of 94.83% was achieved at the end of the study period (n = 55). Fifty four treated teeth showed successful clinical and radiographic outcome at 45 days, 6 months and 12 months (Figure 3). One tooth in Group I reported with signs and symptoms of apical periodontitis and was deemed to be a failure. The three groups were subjected to Fisher's exact test which showed no significant difference (p = 1.000).
The radiographic grey values obtained at the pre-defined follow-up periods in each group were subjected to two-way ANOVA with repeated measures. It revealed that the main effect of groups and the interaction effect (group × time) were not significant with p values of 0.920 and 0.334 respectively. However, the main effect of time was significant (p = 0.0001). At 45 days follow-ups, a significant increase in grey values was observed for group II and III (p < 0.05, Group I: p = 0.16). However, at 6 and 12 months follow-ups, the increase in these values were significant for all the groups (p < 0.05). On inter-group comparison, no significant difference was observed at 45 days, 6 months and 12 months (p > 0.05, Table 1).
A carious lesion is radiographically diagnosed to be 'deep' if it penetrates to a depth of two third or more of the entire dentin thickness.8 The IPT protocol for such lesion includes either one visit or two visit (stepwise excavation) procedures. Orhan et al. conducted a study to assess the outcome of one or two visit IPT in 154 primary and permanent teeth.9 No significant difference was observed in terms of success rate. They concluded that either of the approaches could accomplish the desired outcome. The analysis of clinical studies showed high success rates for both one-visit IPT and stepwise excavation.10,11
Equal distribution of the baseline characteristics, a high recall rate and evaluation by an independent endodontist reduced the outcome bias in the present study. The success/failure of IPT were assessed according to the established guidelines.12 In addition, RVG 'densitometric analysis' of the remaining affected dentin was performed. To date, evidence of remineralization of sealed carious dentin has been demonstrated either by micro-hardness analysis or digital subtraction radiography. The former is an invasive technique requiring re-entry into the cavity. The latter faces the difficulty of image registration. Quantitative radiographic assessment is a valuable attribute of digital diagnostic imaging. It can be categorized into geometric parameters (length, area and volume) and densitometric parameters (tissue mass and density). The basis for two-dimensional radiographic densitometric measurement is that the X-ray attenuation increases with the density of the material for a given thickness.13 The radiographic density is a measure of degree of image darkening and is expressed as grey level numeric values (intensity from 0 - 255). Digital radiography achieves this quantitative analysis using a 'Computer Assisted Densitometric Image Analysis' (CADIA) software. It has been used for detecting subtle changes in the mineralization of hard tissues. Kodak RVG Dental Imaging software 6.12.10.0 incorporates a similar tool. This enables the clinician to quantify the density at desirable points, within the plane and defined fields of the radiogram. Di Alberti et al. studied the peri-implant bone density with a RVG densitometric application.11,14 Using a similar method, Radionov et al. evaluated osseo-integration of a replanted tooth in a nine year old patient.15 They concluded that RVG densitometry showed stable values of bone density.
An increase in radiographic grey values of dentin was observed in every group during the follow-up periods. This can be interpreted as remineralization of the porous and softened dentin. However, on inter-group comparison, this change was not significant at any given follow-up time. The results are in agreement with studies by Franzon et al. who questioned the role/need of a remineralizing agent in IPT and concluded that the increase in the density of remaining dentin was due to a mineral gain resulting from the biological response of the pulp.16,17
Photo-dynamic therapy (PDT) or photo-activated disinfection (PAD) was introduced to dentistry by Professor Michael Wilson in 1993.18 Its use has been extended for eradication of oral pathogenic bacteria that cause caries, endodontic infection, periodontitis and peri-implantitis. It is equally effective against oral gram-positive and negative bacteria. It can kill them in planktonic cultures, plaque scrapings, intact bio-films and dentinal carious lesions.19,20,21,22 Williams et al. studied the efficacy of PAD against Streptococcus mutans in carious dentin and collagen matrix; an environment similar to that which would exist within a carious tooth and concluded that it could achieve appreciable kills (99%).23 It is equally effective against other cariogenic microorganisms including Streptococcus sobrinus, Lactobacillus casei and Actinomyces viscosus.6
PAD solution is tolonium chloride which is a pharmaceutical grade of 'toluidine blue O' and belongs to the phenothiazinium class of photo-sensitizer (PS) compounds. The dye is amphiphilic in nature and this is responsible for its effective penetration in both gram positive and negative micro-organisms. It has a dye concentration of 0.01 mg/mL. Above this, formation of dye 'self-aggregates' (dimeric form) occurs and modifies its absorption spectrum.24 Hence the monomeric form was preferred. A laser of 635 nm was utilized as it matches the absorption spectrum of the dye.
The bactericidal action of PAD is a consequence of PS and light interaction. The excited PS molecule interacts with the endogenous oxygen of the target cells via two pathways. The Type I involve electron-transfer reaction resulting in the formation of toxic oxygen species such as superoxide, hydroxyl radicals and hydrogen peroxide. The Type II process entails energy transfer from the PS triplet state to ground state molecular oxygen, resulting in production of excited singlet oxygen (1O2). These reactive species induces DNA and cytoplasmic membrane damage leading to bacterial cell death.18
It is desirable to have a short exposure time for clinical convenience. Williams et al. demonstrated that energy dose of 1.8 Joules or more killed 100% of the carious bacteria present.7 This can be adjusted and calculated by multiplying the exposure time (second) and the laser output power (mW). An energy dose of 6 Joules (60 seconds × 100 mW, 1 mW = 0.001 J/sec) was utilized in this study. This was sufficient for a profound antibacterial action and safe for pulp vitality.25
The proposed hypothesis was sustained based on the success of IPT achieved in all groups. The results suggest that PAD and CH both have equal disinfection efficacy in the treatment of deep carious dentin. PAD probably provided an effective and convenient method for reducing the microbial load of the remaining carious dentin. Clinically, this would make it less critical to distinguish between infected and affected dentin. 'Favorable conditions' for healing of the pulp-dentin complex can be achieved without the placement of a remineralizing material.
The present pilot clinical work was based on the premise that PAD is a highly effective antibacterial treatment modality. However, further in vivo microbiological investigations need to be conducted to establish the efficacy of PAD for disinfection of deeper layers of carious dentin. Usefulness of 'RVG Densitometry' as a viable tool for measuring relative density of dentin needs further investigation as well. To positively substantiate the results of the present study and establish PAD in the treatment protocol of IPT, longitudinal studies with larger sample size would need to be undertaken.
PAD and CH both have equal disinfection efficacy in the treatment of deep carious dentin. PAD alone is as effective for treatment of deep carious lesion as CH and hence can be used as an alternative to CH. They can be used independently in IPT, since combining both does not offer any additional therapeutic benefits.
Acknowledgement
The authors would like to thank the patients for participating in the present study. All the materials were provided by the institution.
References
1. Ingle JI, Bakland LK. Endodontics. 6th ed. London: DC Becker Inc;2008. p. 1310–1312.
2. Fusayama T, Okuse K, Hosoda H. Relationship between hardness, discoloration, and microbial invasion in carious dentin. J Dent Res. 1966; 45:1033–1046. PMID: 5224073.

3. Fagundes TC, Barata TJ, Prakki A, Bresciani E, Pereira JC. Indirect pulp treatment in a permanent molar: case report of 4-year follow-up. J Appl Oral Sci. 2009; 17:70–74. PMID: 19148410.
4. Eidelman E, Finn SB, Koulourides T. Remineralization of carious dentin treated with calcium hydroxide. J Dent Child. 1965; 32:218–225. PMID: 5318013.
5. Pinto AS, de Araújo FB, Franzon R, Figueiredo MC, Henz S, García-Godoy F, Maltz M. Clinical and microbiological effect of calcium hydroxide protection in indirect pulp capping in primary teeth. Am J Dent. 2006; 19:382–386. PMID: 17212082.
6. Burns T, Wilson M, Pearson GJ. Sensitisation of cariogenic bacteria to killing by light from helium-neon laser. J Med Microbiol. 1993; 38:401–405. PMID: 8510132.
7. Williams JA, Pearson GJ, Colles MJ, Wilson M. The effect of variable energy input from a novel light source on the photo-activated bactericidal action of toluidine blue O on Streptococcus mutans. Caries Res. 2003; 37:190–193. PMID: 12740542.
8. Gruythuysen RJ, van Strijp AJ, Wu MK. Long-term survival of indirect pulp treatment performed in primary and permanent teeth with clinically diagnosed deep carious lesions. J Endod. 2010; 36:1490–1493. PMID: 20728715.

9. Orhan AI, Oz FT, Orhan K. Pulp exposure occurrence and outcomes after 1- or 2-visit indirect pulp therapy vs complete caries removal in primary and permanent molars. Pediatr Dent. 2010; 32:347–355. PMID: 20836956.
10. Al-Zayer MA, Straffon LH, Feigal RJ, Welch KB. Indirect pulp treatment of primary posterior teeth: a retrospective study. Pediatr Dent. 2003; 25:29–36. PMID: 12627699.
11. Bjørndal L, Larsen T, Thylstrup A. A clinical and microbiological study of deep carious lesions during stepwise excavation using long treatment intervals. Caries Res. 1997; 31:411–417. PMID: 9353579.

12. Evidence-based review of clinical studies on indirect pulp capping. J Endod. 2009; 35:1147–1151. Edited by Gutmann JL, Solomon E. PMID: 19631852.
13. Park YS, Bae KH, Chang J, Shon WJ. Theory of X-ray microcomputed tomography in dental research: application for the caries research. J Korean Acad Conserv Dent. 2011; 36:98–107.

14. Di Alberti L, Donnini F, Di Alberti C, Camerino M. A comparative study of bone densitometry during osseointegration: piezo-electric surgery versus rotary protocols. Quintessence Int. 2010; 41:639–644. PMID: 20657852.
15. Radionov D, Lulic-Dukić L, Gasparac I. Osseointegration of a replanted tooth followed by RVG densitometry. Coll Antropol. 1998; 22(Suppl):161–166. PMID: 9951158.
16. Franzon R, Casagrande L, Pinto AS, García-Godoy F, Maltz M, de Araujo FB. Clinical and radiographic evaluation of indirect pulp treatment in primary molars: 36 months follow-up. Am J Dent. 2007; 20:189–192. PMID: 17672262.
17. Franzon R, Gomes M, Pitoni CM, Bergmann CP, Araujo FB. Dentin rehardening after indirect pulp treatment in primary teeth. J Dent Child (Chic). 2009; 76:223–228. PMID: 19941765.
18. Soukos NS, Goodson JM. Photodynamic therapy in the control of oral biofilms. Periodontol 2000. 2011; 55:143–166. PMID: 21134233.

19. Soukos NS, Ximenez-Fyvie LA, Hamblin MR, Socransky SS, Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob Agents Chemother. 1998; 42:2595–2601. PMID: 9756761.

20. Wilson M, Burns T, Pratten J, Pearson GJ. Bacteria in supra-gingival plaque samples can be killed by low-power laser light in the presence of a photosensitizer. J Appl Bacteriol. 1995; 78:569–574. PMID: 7759386.

21. Wood S, Nattress B, Kirkham J, Shore R, Brookes S, Griffiths J, Robinson C. An in vitro study of the use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo. J Photochem Photobiol B. 1999; 50:1–7. PMID: 10443029.
22. Giusti JS, Santos-Pinto L, Pizzolito AC, Helmerson K, Carvalho-Filho E, Kurachi C, Bagnato VS. Antimicrobial photodynamic action on dentin using a light-emitting diode light source. Photomed Laser Surg. 2008; 26:281–287. PMID: 18637719.

23. Williams JA, Pearson GJ, Colles MJ, Wilson M. The photo-activated antibacterial action of toluidine blue O in a collagen matrix and in carious dentin. Caries Res. 2004; 38:530–536. PMID: 15528907.
24. Nagata JY, Hioka N, Kimura E, Batistela VR, Terada RS, Graciano AX, Baesso ML, Hayacibara MF. Antibacterial photodynamic therapy for dental caries: evaluation of the photosensitizers used and light source properties. Photodiagnosis Photodyn Ther. 2012; 9:122–131. PMID: 22594982.

25. Nammour S, Zeinoun T, Bogaerts I, Lamy M, Geerts SO, Bou Saba S, Lamard L, Peremans A, Limme M. Evaluation of dental pulp temperature rise during photo-activated decontamination (PAD) of caries: an in vitro study. Lasers Med Sci. 2010; 25:651–654. PMID: 19488675.
Figure 1
(a) Preoperative; (b) After caries excavation; (c) PAD solution application; (d) PAD light application.
PAD, photo-activated disinfection.
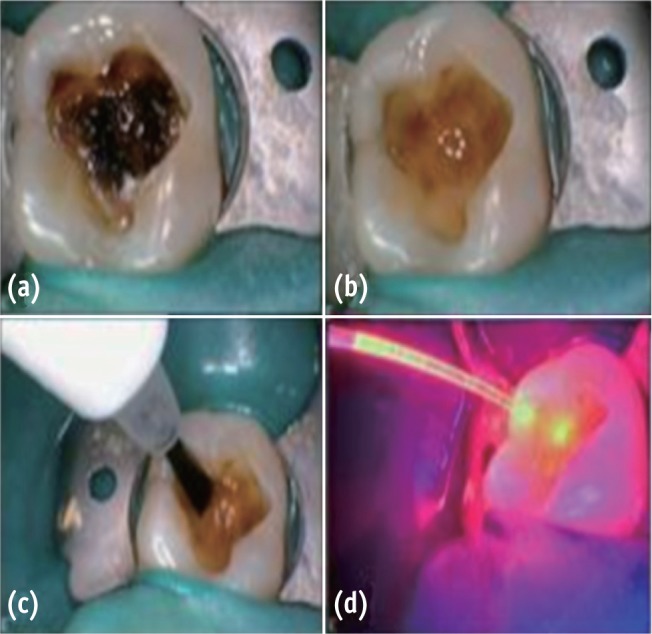
Figure 2
Densitometric analysis of remaining affected dentin after IPT.
IPT, indirect pulp treatment.
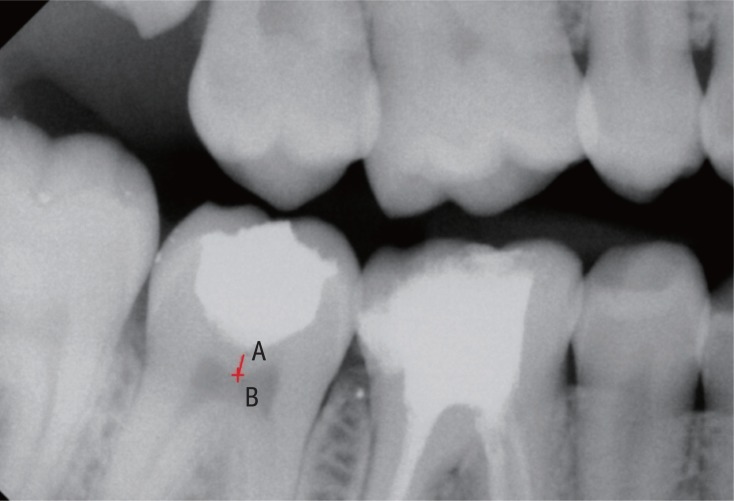
Figure 3
Group I, PAD; Group II, CH; Group III, PAD + CH. (a) Preoperative; (b) Postoperative; (c) At 45 days after treatment; (d) At 6 months after treatment; (e) At 12 months after treatment.
PAD, photo-activated disinfection; CH, calcium hydroxide.
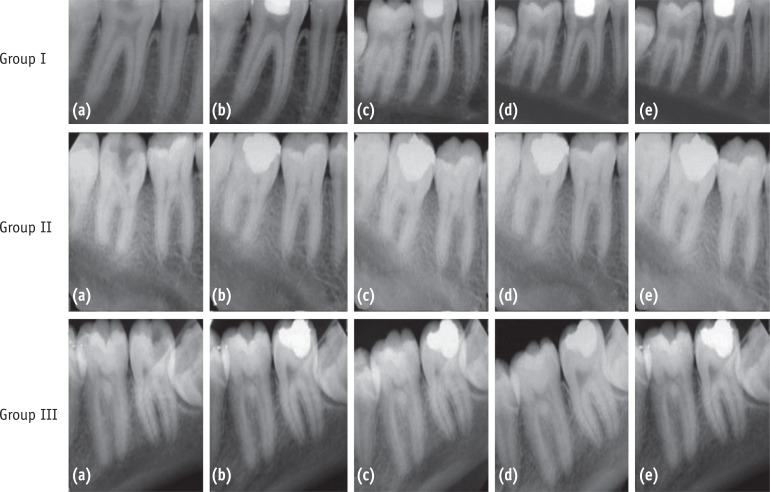
Table 1
Radiographic grey values of remaining affected dentin in Groups I, II, and III

Different superscript lower-case letters indicate a statistically significant difference between different time points for each group (within the row, p < 0.05) and different superscript upper-case letters indicate a statistically significant difference among the groups (within the column, p < 0.05).
PAD, photo-activated disinfection; CH, calcium hydroxide.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download