Abstract
Objectives
This study aimed to assess the effect of 38% carbamide peroxide on the microleakage of class V cavities restored with either a silorane-based composite or two methacrylate-based composites.
Materials and Methods
A total of 96 class V cavities were prepared on the buccal surface of extracted human teeth with both enamel and dentin margins and were randomly assigned into three groups of Filtek P90 (3M-ESPE) + P90 system adhesive (3M-ESPE)(group A), Filtek Z250 (3M-ESPE) + Adper Prompt L-Pop (3M-ESPE)(group B) and Filtek Z350XT (3M-ESPE) + Adper Prompt L-Pop (group C). Half of the teeth were randomly underwent bleaching (38% carbamide peroxide, Day White, Discus Dental, applying for 15 min, twice a day for 14 day) while the remaining half (control) were not bleached. Dye penetration was measured following immersion in basic fuchsine. Data were statistically analyzed using Kruskal-Wallis and Mann-Whitney U tests at a level of 0.05.
Results
No significant differences were found between composites in the control groups in enamel (p = 0.171) or dentin (p = 0.094) margins. After bleaching, microleakage of Z250 (in enamel [p = 0.867] or dentin [p = 0.590] margins) and Z350 (in enamel [p = 0.445] or dentin [p = 0.591] margins) did not change significantly, but the microleakage of P90 significantly increased in both enamel (p = 0.042) and dentin (p = 0.002) margins.
Tooth-colored restorations, especially composite resins are now part of contemporary dentistry.1,2 However, drawbacks such as polymerization shrinkage have compromised their clinical success. Polymerization shrinkage will cause gap at the tooth-restoration interface and subsequent microleakage, which allows the passage of fluids, bacteria and ions leading to the development of complications such as hypersensitivity, pulpal irritation and marginal discoloration.2,3,4 The following approaches may help to reduce the polymerization shrinkage and the resulting stress: deceleration of polymerization rate, replacement of dual-cured composites with self-cured resins, applying a thicker adhesive coat below the composite resin and application of incremental technique.5,6,7,8 Changing the resin matrix and production of composite resins with small polymerization shrinkage such as silorane-based composites is a recently proposed technique to reduce the polymerization shrinkage.2 These composites undergo cationic ring-opening polymerization.2,4 New monomers are produced by the reaction of oxirane and siloxane molecules and the name 'silorane' is derived from the names of these two molecules.2,3 The manufacturer claims that this composite resin has two main advantages: the first is its small polymerization shrinkage due to the oxirane ring opening mechanism and the second is its increased hydrophobicity attributed to the presence of siloxane.4 Studies have demonstrated that silorane-based composites have similar or even more favorable mechanical and physical characteristics compared to methacrylate-based composites: polymerization shrinkage less than 1.5%, low water sorption, optimal biocompatibility, suitable color stability and good marginal fit.9,10,11,12,13,14,15
Bleaching is an effective and relatively safe esthetic treatment.16,17 The bleaching agent usually contains peroxide (such as hydrogen peroxide, carbamide peroxide, and sodium perborate) and is usually applied through the office- or home-bleaching techniques.16,18,19 Office bleaching is usually carried out with the use of 35 - 38% hydrogen peroxide applied to the tooth surface for 30 - 45 minutes. This process may be activated by light. Home bleaching is usually done with the application of carbamide peroxide delivered in a special tray customized for the patient; which is usually used at night.16
Some researchers have investigated the effect of bleaching agents on physicochemical characteristics of tooth structure and some others have evaluated the influence of bleaching materials on the properties of methacrylate-based composite resins such as the elution of methacrylate monomers, their surface hardness and roughness, color and microleakage.17,18,19,20,21,22 The impact of whitening agents on the surface roughness and hardness and enamel-dentin bond strength of silorane-based composite resins has been evaluated in the literature as well.16,23 A group of researchers believe that tooth whitening agents are able to penetrate into the tooth structure through the unsealed dentin margin at the tooth-restoration interface and thus, are capable of causing complications like tooth hypersensitivity and microleakage.17,24 However, to date, no study has evaluated the effect of bleaching agents on the microleakage of cavities restored with silorane-based composite resins. On the one hand, Filtek Z350 is a nano-filled composite which has been claimed to have a low shrinkage due to its high filler volume.25 Also, Z250 has had acceptable microleakage results in previous studies.26,27 Some manufacturers have produced home bleaching agents with higher concentrations of carbamide peroxide and shorter overnight application time that may have different effects compared to 10 - 15% carbamide peroxide. Therefore, the present study sought to assess the effect of a highly concentrated home bleaching agent on the microleakage of class V cavities restored with silorane-based composite resins in comparison with two methacrylate-based composites (microhybrid and nanofilled). The null hypothesis is that the effect of bleaching with 38% carbamide peroxide on the microleakage of silorane-based and methacrylate-based composite restorations is the same.
The name of products used, their composition and their manufacturing companies are summarized in Table 1. Three types of restorative materials were used in this study: a methacrylate-based microhybrid composite resin (Filtek Z250, 3M-ESPE, St. Paul, MN, USA) and Adper Prompt L-Pop bonding (self-etch adhesive, 3M-ESPE), a methacrylate-based nanofilled composite resin (Filtek Z350, 3M-ESPE) and Adper Prompt L-Pop bonding, and a silorane-based composite resin (Filtek P90, 3M-ESPE) and self-etch bonding and primer (P90 System adhesive, 3M-ESPE). Day white home bleaching system (Discus Dental, Culver City, CA, USA) containing 38% carbamide peroxide was used for bleaching.
A total of 96 sound premolar teeth that had recently been extracted as part of orthodontic treatment plan and were free from carious lesions, cracks, fracture, or restorations were selected. The teeth were cleaned from blood and tissue appendages and immersed in 0.5% chloramine T solution at 4℃ for one week according to ISO 11405 standards. Then the teeth were polished with a rotary bristle brush and pumice paste and kept in distilled water to be used for the experiment within the next 3 months. Standard class V cavities measuring 3 mm (mesiodistal) × 2 mm (occlusogingival) × 1.5 mm (depth) were prepared by a diamond 008 fissure bur (Stoddard manufacturing, Garden City, England) and high speed hand piece with water spray on the buccal surface of all teeth with gingival margin 1 mm below the cementoenamel junction. The bur was changed after preparation of each 5 samples.
The teeth were randomly assigned into three groups as follows:
For group A, PLP was mixed according to the manufacturer's instructions and the entire surface of the cavity was coated with the bonding agent using a microbrush and scrubbed for 15 seconds. After gentle air drying for 5 seconds to thin the adhesive coat, the entire surface was coated with the second layer of adhesive. After gentle air drying for 10 seconds, the adhesive layer was cured with the LED light curing unit (Valo, Ultradent Products Inc., South Jordan, UT, USA) with 1,000 mW/cm2 intensity. Z250 composite resin was incrementally applied to the cavity obliquely in three layers. The first layer of composite resin was applied to the occlusal margin and occlusal one-third of axial wall, the second layer was applied to the middle one-third of the axial wall and the third layer to the gingival margin and gingival one-third of the axial wall. Each layer was photo cured for 10 seconds.
Group B specimens were prepared according to the same protocol used for the previous group. Group C specimens were prepared according to the manufacturer's instructions as follows: the entire surface of the cavity was coated with primer using a microbrush and scrubbed for 15 seconds, followed by gentle air drying and light curing with an LED light-curing unit with 1,000 mW/cm2 intensity for 10 seconds. By using another microbrush, the entire surface of the cavity was evenly soaked with the bonding agent, followed by gentle air drying and light curing for 10 seconds. The cavities were restored with P90 composite resin with the same technique described earlier. It should be mentioned that the intensity of the light curing unit was checked after curing every 8 specimens by a radiometer. The specimens were polished by using Soflex aluminum oxide polishing discs (Coarse, medium, fine, super fine, 3M-ESPE) and post-cured for another 20 seconds. The teeth were then immersed in distilled water and stored at 37℃ for 24 hours.
Specimens were subjected to thermocycling for 1,000 cycles between 5℃ and 55℃ (± 2℃), with a 30 seconds dwell time and a 10 seconds transfer time and then stored in 37℃ distilled water.
Each group was randomly divided into two subgroups of 16 each (A1, A2, B1, B2, C1, and C2). A1, B1 and C1 were immersed in distilled water at 37℃ as the control groups and did not undergo the bleaching process. The teeth in A2, B2 and C2 groups were prepared for bleaching as follows: the teeth were first dried and separately fixed on a piece of dental wax on a smooth surface. According to the manufacturer's instructions, 38% carbamide peroxide (Day White, Discus Dental) was applied twice a day for 15 minutes for a duration of 2 weeks. At each cycle, the restoration surface of samples was covered with the whitening gel, placed in a dark environment for 15 minutes. Samples were then rinsed with water and stored in distilled water at 37℃.
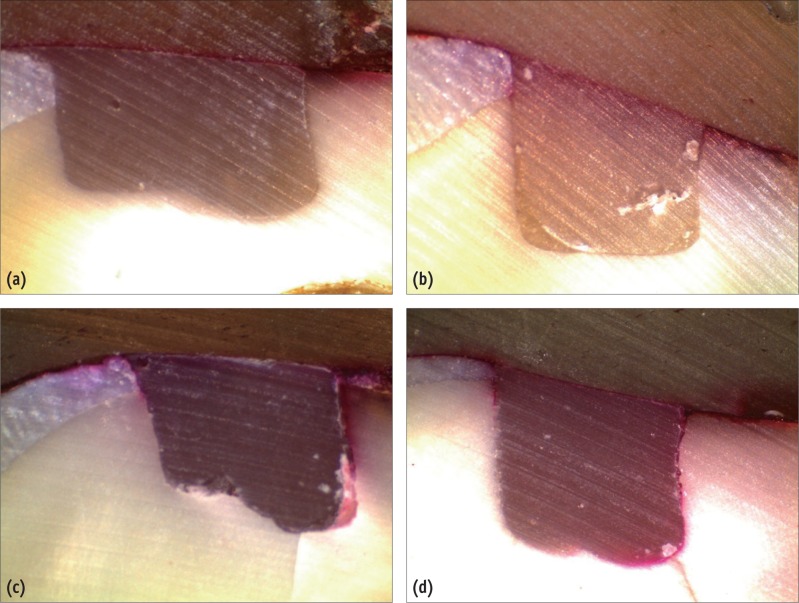
Apex, root and furcation area were sealed by a layer of adhesive wax. All tooth surfaces were sealed with 2 layers of nail polish except for the restored area and 1 mm around it. The specimens were then immersed in 2% basic fuchsine solution and stored in an incubator at 37℃ for 24 hours. The teeth were then washed and dried with sterile gauze and air spray. Each tooth was separately mounted in a special mold using clear polyester resin. After that they were longitudinally sectioned by a cutting machine with 0.82 mm sectioning blade thickness (Mecatome T201A, Presi, Brié-et-Angonnes, France). Gingival and occlusal marginal microleakage was assessed under a stereomicroscope (Nikon, Tokyo, Japan) with ×40 magnification and scored as below (Figure 1):
Kruskal-Wallis test was used to compare the three composites. If it was significant, Mann Whitney U test with boneferroni adjustment was used for comparisons of two. Whitney U test was used for the comparison of bleached and control subgroups of each composite as well. p < 0.05 was considered statistically significant.
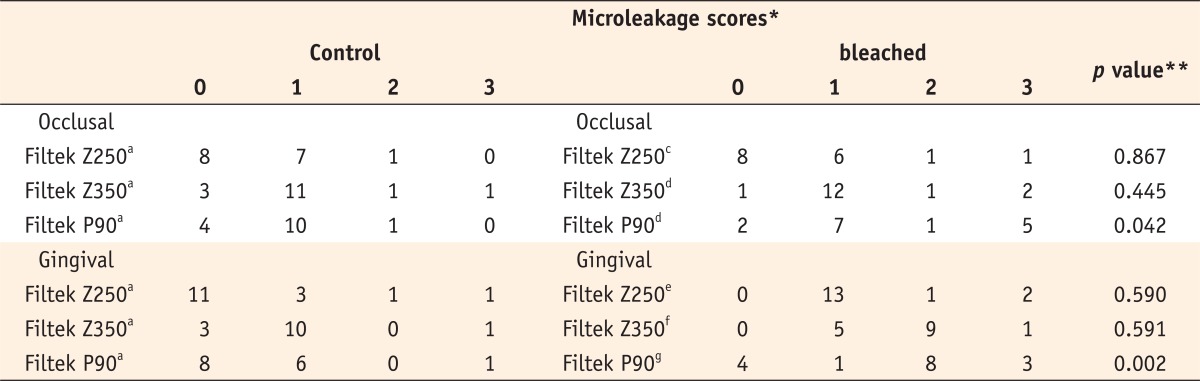
Microleakage scores are given in Table 2. Kruskal-Wallis test indicated no significant differences between the three control groups of Z250, Z350 and P90 composite resins at the enamel (p = 0.171) or dentin (p = 0.094) margins. After the application of 38% carbamide peroxide, the comparison between the three studied groups in Mann-Whitney U test showed that the microleakage of Z350 was significantly higher than that of Z250 at both enamel (p = 0.024) and dentin (p = 0.027) margins. P90 also showed significantly higher microleakage than Z250 at both enamel (p = 0.024) and dentin (p < 0.001) margins. It showed a greater microleakage than Z350 at the dentin margin (p = 0.014), but no significant difference was observed at the enamel margin (p = 0.47).
Mann-Whitney U test showed no significant differences in the microleakage of Z250 composite in enamel (p = 0.867) or dentinal (p = 0.590) margins compared to the control group. Also, no significant differences were found in the microleakage of Z350 composite in enamel (p = 0.445) or dentinal (p = 0.591) margins compared to the control group. However, the microleakage of P90 composite restorations significantly increased in both enamel (p = 0.042) and dentin (p = 0.002) margins compared to the control group.
No significant differences were noted in the microleakage between the enamel and dentin margins of P90 (p = 0.863) and Z250 (p = 0.058), but the microleakage of Z350 at enamel margin was significantly greater than dentin margin (p = 0.011).
Dye penetration is the most commonly used technique for the assessment of the sealability of dental materials under in vitro conditions.28 In the oral cavity, dental materials are subjected to thermal changes and the difference in the modulus of thermal conductivity of the tooth and restorative materials causes stress at the interface.29 Thermocycling has been used in the present study to simulate these changes in order to evaluate the effect of bleaching on dental restorations in the mouth. The bond strength of composite to enamel is not as challenging as to dentin.30 Therefore, we have prepared standard class V cavities with occlusal margins located in enamel and gingival margins in dentin, to evaluate the effect of bleaching on microleakage in both enamel and dentin. Bleaching agents are extremely unstable, therefore they produce free radicals that lead to the cleavage of polymer chains and break the double bonds. Additionally, hydrogen peroxide is diffusible.16,31
In the present study, the microleakage of the control group of P90 was not significantly different with the two other control groups in methacrylate-based composites. Similar to our study, Palin et al. compared the microleakage and cuspal deflection of MOD cavities filled with EXL596 and Silorane H1 composites, and Filtek Z250 and Z100 methacrylate based composites.32 They found no significant differences between the microleakage of Silorane H1, Filtek Z250 and Z100. Nevertheless, another study done by Hooshmand et al. reported lower microleakage of silorane based composites compared to Tetric Evo Ceram and Tetric Ceram.33 There is also another study done by Roula Al-Boni et al. which has also shown that class 1 cavities filled with silorane based composites had a lower microleakage compared to Filtek Z250 and Amelogen Plus methacrylate based composites.34 These controversial results in the mentioned studies might have been caused by the kind of material or method used for the tests.
No significant difference was observed in microleakage after application of 38% carbamide peroxide for 15 minutes twice a day between the two methacrylate-based composites and the control groups at both the dentin and enamel margins. Interestingly it was observed that the microleakage of P90 increased after bleaching compared to the control group, but unfortunately there has not been any previous researches available to compare the results with. In a study done by White et al. which analyzed the effects of bleaching agents on the microleakage of class 5 cavities filled with Filtek Z250, they found no significant differences among 20% carbamide proxide, 6% hydrogen proxide and the control group.35 Nevertheless, Moosavi et al., have compared the effects of 14% carbamide proxide bleaching agent on class 5 cavities filled by Filtek P60 and RMGI in different time periods and found that the microleakage in P60 dentin margin and RMGI enamel margin significantly increased after bleaching compared to the control group.36
After bleaching with 38% carbamide peroxide, the microleakage in the dentin margin of P90 was greater than that of Z350. The score of microleakage in both margins of P90 was higher than the corresponding value in Z250. A possible explanation for this observation might be related to the properties of their adhesive system. P90 is applied along with a two-step self-etch adhesive commercially named P90 System Adhesive (PSA). First, a self-etching hydrophilic primer (PSA self-etch primer) is applied and separately cured. Then, a hydrophobic adhesive resin (PSA bond) is applied. PSA bond is methacrylate-based and compatible with conventional methacrylate composites.37 This adhesive system has been designed to bridge the gap between the hydrophilic dentin and hydrophobic silorane-based composite. P90 primer and bonding agent are provided in separate bottles and are separately photo-activated, whereas primer and bonding agent of the other two-step self-etch adhesives are mixed on the dentin surface and are photo-cured simultaneously.2 A previous study has shown less microleakage in P90 compared to Z250.34 Yamazaki et al. has also demonstrated that the microleakage of P90 was less than that of Tetric Ceram after loading but no significant differences were noted without loading.38 In contrast, Palin et al. reported that application of silorane-based composite resins in class V restorations did not lead to the development of microleakage in comparison to methacrylate-based composites.32 D'Alpino et al. revealed that the self-etch bonding systems especially that of P90 had a smaller interfacial gap compared to etch and rinse adhesives.2 Tezvergil-Mutluay et al. has shown that silorane adhesive has had the least microleakage even in association with a methacrylate-based composite which can be due to the high viscosity of the two-layer filled P90 adhesive and it forms a thick adhesive layer on the tooth surface.39 But the bond longevity of P90 is of great concern.40 Although, according to the manufacturer's claim, a slight demineralization of tooth structure happens due to the low pH of P90 primer (2.7), we did not see a durable bond after the bleaching of P90.2 On the other hand, although we know that PLP is a low-pH self-etch adhesive (0.4), since its primer and adhesive are being applied all at one step, the infiltration of the adhesive is quite equal to the depth of demineralization which has been created by primer.41 The reason why bleaching in this study did not change the microleakage of restorations can be ascribed to the bond with PLP. It has been claimed and recently been confirmed that P90 primer forms a chemical bond with hydroxyapatite crystals.42 It has been documented that optimal bond stability is achievable in silorane-based adhesives. However, there have been concerns regarding the quality and long-term stability of the P90 adhesive hybrid layer.40 Santini and Miletic have observed an intermediate zone with 1 micrometer thickness between the primer and the silorane bond with the use of Micro Raman spectroscopy.43 The authors of the mentioned study believed that this area might be the weakest zone of silorane adhesives which causes failure in restorations and further investigations are required in this respect. Considering the claims regarding the presence of chemical bond and one micrometer intermediate zone, it appears that the destructive effects of free radicals released by the carbamide peroxide led to increased microleakage in silorane-based composite resin in the present study. However, we recommend further investigations to evaluate the degradation process of silorane-based composite.
References
1. Mujdeci A, Gokay O. Effect of bleaching agents on the microhardness of tooth-colored restorative materials. J Prosthet Dent. 2006; 95:286–289. PMID: 16616125.

2. D'Alpino PH, Bechtold J, dos Santos PJ, Alonso RC, Di Hipólito V, Silikas N, Silikas N, Rodrigues FP. Methacrylate- and silorane-based composite restorations: hardness, depth of cure and interfacial gap formation as a function of the energy dose. Dent Mater. 2011; 27:1162–1169. PMID: 21925724.
3. Bagis YH, Baltacioglu IH, Kahyaogullari S. Comparing microleakage and the layering methods of silorane-based resin composite in wide Class II MOD cavities. Oper Dent. 2009; 34:578–585. PMID: 19830973.

4. Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater. 2005; 21:68–74. PMID: 15681004.

5. Mehl A, Hickel R, Kunzelmann KH. Physical properties and gap formation of light-cured composites with and without 'softstart-polymerization'. J Dent. 1997; 25:321–330. PMID: 9175364.

6. Braga RR, Ferracane JL, Condon JR. Polymerization contraction stress in dual-cure cements and its effect on interfacial integrity of bonded inlays. J Dent. 2002; 30:333–340. PMID: 12554115.

7. Choi KK, Condon JR, Ferracane JL. The effects of adhesive thickness on polymerization contraction stress of composite. J Dent Res. 2000; 79:812–817. PMID: 10765953.

8. Lutz E, Krejci I, Oldenburg TR. Elimination of polymerization stresses at the margins of posterior composite resin restorations: a new restorative technique. Quintessence Int. 1986; 17:777–784. PMID: 3468527.
9. Ilie N, Hickel R. Silorane-based dental composite: behavior and abilities. Dent mater J. 2006; 25:445–454. PMID: 17076313.

10. Lien W, Vandewalle KS. Physical properties of a new silorane-based restorative system. Dent Mater. 2010; 26:337–344. PMID: 20053434.

11. Eick JD, Smith RE, Pinzino CS, Kostoryz EL. Stability of silorane dental monomers in aqueous systems. J Dent. 2006; 34:405–410. PMID: 16288948.

12. Schweikl H, Schmalz G, Weinmann W. The induction of gene mutations and micronuclei by oxiranes and siloranes in mammalian cells in vitro. J Dent Res. 2004; 83:17–21. PMID: 14691107.
13. Pires-de-Souza Fde C, Garcia Lda F, Roselino Lde M, Naves LZ. Color stability of silorane-based composites submitted to accelerated artificial ageing-an in situ study. J Dent. 2011; 39(Supplment 1):e18–e24. PMID: 21421020.
14. Schmidt M, Kirkevang LL, Hørsted-Bindslev P, Poulsen S. Marginal adaptation of a low-shrinkage silorane-based composite: 1-year randomized clinical trial. Clin Oral Investig. 2011; 15:291–295.

15. Santos PJ, Silva MS, Alonso RC, D'Alpino PH. Hydrolytic degradation of silorane- and methacrylate-based composite restorations: evaluation of push-out strength and marginal adaptation. Acta Odontol Scand. 2013; 71:1273–1279. PMID: 23394207.

16. Atali PY, Topbaşi FB. The effect of different bleaching methods on the surface roughness and hardness of resin composites. J Dent Oral Hyg. 2011; 3:10–17.
17. Khoroushi M, Fardashtaki SR. Effect of light-activated bleaching on the microleakage of Class V tooth-colored restorations. Oper Dent. 2009; 34:565–570. PMID: 19830971.

18. Polydorou O, Beiter J, König A, Hellwig E, Kümmerer K. Effect of bleaching on the elution of monomers from modern dental composite materials. Dent Mater. 2009; 25:254–260. PMID: 18774601.

19. Sharafeddin F, Jamalipour G. Effects of 35% carbamide peroxide gel on surface roughness and hardness of composite resins. J Dent (Tehran). 2010; 7:6–12. PMID: 21998769.
20. Attin T, Schmidlin PR, Wegehaupt F, Wiegand A. Influence of study design on the impact of bleaching agents on dental enamel microhardness: a review. Dent Mater. 2009; 25:143–157. PMID: 18635255.

21. El-Murr J, Ruel D, St-Georges AJ. Effects of external bleaching on restorative materials: a review. J Can Dent Assoc. 2011; 77:b59. PMID: 21627869.
22. Anagnostou M, Chelioti G, Chioti S, Kakaboura A. Effect of tooth-bleaching methods on gloss and color of resin composites. J Dent. 2010; 38(Supplment 2):e129–e136. PMID: 20600560.

23. Lima AF, Sasaki RT, Araújo LS, Gaglianone LA, Freitas MS, Aguiar FH, Marchi GM. Effect of tooth bleaching on bond strength of enamel-dentin cavities restored with silorane- and dimethacrylate-based materials. Oper Dent. 2011; 36:390–396. PMID: 21827224.

24. Attin T, Hannig C, Wiegand A, Attin R. Effect of bleaching on restorative materials and restorations-a systematic review. Dent Mater. 2004; 20:852–861. PMID: 15451241.

25. Pereira RA, Araujo PA, Castañeda-Espinosa JC, Mondelli RF. Comparative analysis of the shrinkage stress of composite resins. J Appl Oral Sci. 2008; 16:30–34. PMID: 19089286.

26. Sharma RD, Sharma J, Rani A. Comparative evaluation of marginal adaptation between nanocomposites and microhybrid composites exposed to two light cure units. Indian J Dent Res. 2011; 22:495. PMID: 22048600.

27. Hardan LS, Amm EW, Ghayad A, Ghosn C, Khraisat A. Effect of different modes of light curing and resin composites on microleakage of Class II restorations-Part II. Odontostomatol Trop. 2009; 32:29–37. PMID: 20069964.
28. Raskin A, D'Hoore W, Gonthier S, Degrange M, Déjou J. Reliability of in vitro microleakage tests: a literature review. J Adhes Dent. 2001; 3:295–308. PMID: 11893045.
29. Hilton TJ. Can modern restorative procedures and materials reliably seal cavities? In vitro investigations. Part 2. Am J Dent. 2002; 15:279–289. PMID: 12572649.
30. Swift EJ Jr, Perdigão J, Heymann HO. Bonding to enamel and dentin: a brief history and state of the art. Quintessence Int. 1995; 26:95–110. PMID: 7568728.
31. Malkondu Ö, Yurdagüven H, Say EC, Kazazoğlu E, Soyman M. Effect of bleaching on microhardness of esthetic restorative materials. Oper Dent. 2011; 36:177–186. PMID: 21702674.

32. Palin WM, Fleming GJ, Nathwani H, Burke FJ, Randall RC. In vitro cuspal deflection and microleakage of maxillary premolars restored with novel low-shrink dental composites. Dent Mater. 2005; 21:324–335. PMID: 15766579.
33. Hooshmand T, Tabari N, Keshvad A. Marginal leakage and microhardness evaluation of low-shrinkage resin-based restorative materials. Gen Dent. 2013; 61:46–50. PMID: 23302363.
34. Al-Boni R, Raja OM. Microleakage evaluation of silorane based composite versus methacrylate based composite. J Conserv Dent. 2010; 13:152–155. PMID: 21116392.

35. White DJ, Duschner H, Pioch T. Effect of bleaching treatments on microleakage of Class I restorations. J Clin Dent. 2008; 19:33–36. PMID: 18500158.
36. Moosavi H, Ghavamnasiri M, Manari V. Effect of postoperative bleaching on marginal leakage of resin composite and resin-modified glass ionomer restorations at different delayed periods of exposure to carbamide peroxide. J Contemp Dent Pract. 2009; 10:E009–E016.

37. Van Ende A, De Munck J, Mine A, Lambrechts P, Van Meerbeek B. Does a low-shrinking composite induce less stress at the adhesive interface? Dent Mater. 2010; 26:215–222. PMID: 19906417.

38. Yamazaki PC, Bedran-Russo AK, Pereira PN, Swift EJ Jr. Microleakage evaluation of a new low-shrinkage composite restorative material. Oper Dent. 2006; 31:670–676. PMID: 17153975.

39. Tezvergil-Mutluay A, Lassila LV, Vallittu PK. Incremental layers bonding of silorane composite: the initial bonding properties. J Dent. 2008; 36:560–563. PMID: 18467017.

40. Navarra CO, Cadenaro M, Armstrong SR, Jessop J, Antoniolli F, Sergo V, Di Lenarda R, Breschi L. Degree of conversion of Filtek Silorane Adhesive System and Clearfil SE Bond within the hybrid and adhesive layer: an in situ Raman analysis. Dent Mater. 2009; 25:1178–1185. PMID: 19570569.
41. Summitt JB, Robbins JW, Hilton TJ, Schwartz RS, dos Santos J Jr. Fundamentals of operative dentistry: a contemporary approach. 3rd ed. Illinois: Quintessence Pub. Co.;2006. p. 204. p. 221.
42. Mine A, De Munck J, Van Ende A, Cardoso MV, Kuboki T, Yoshida Y, Van Meerbeek B. TEM characterization of a silorane composite bonded to enamel/dentin. Dent Mater. 2010; 26:524–532. PMID: 20202675.

43. Santini A, Miletic V. Comparison of the hybrid layer formed by Silorane adhesive, one-step self-etch and etch and rinse systems using confocal micro-Raman spectroscopy and SEM. J Dent. 2008; 36:683–691. PMID: 18550251.

Figure 1
Representative microscopic images showing the scores of microleakage, taken under a stereomicroscope (at a ×40 magnification). (a) score 0; (b) score 1; (c) score 2; (d) score 3.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download