Abstract
This article presents the successful surgical management of a failed mineral trioxide aggregate (MTA) orthograde obturation of a tooth with a history of impact trauma and perforated internal root resorption. A symptomatic maxillary lateral incisor with a history of perforation due to internal root resorption and nonsurgical repair using MTA was referred. Unintentional overfill of the defect with MTA had occurred 4 yr before the initial visit. The excess MTA had since disappeared, and a radiolucent lesion adjacent to the perforation site was evident radiographically. Surgical endodontic retreatment was performed using calcium enriched mixture (CEM) cement as a repair material. Histological examination of the lesion revealed granulation tissue with chronic inflammation, and small fragments of MTA encapsulated within fibroconnective tissue. At the one and two year follow up exams, all signs and symptoms of disease had resolved and the tooth was functional. Complete radiographic healing of the lesion was observed two years after the initial visit. This case report illustrates how the selection of an appropriate approach to treatment of a perforation can affect the long term prognosis of a tooth. In addition, extrusion of MTA into a periradicular lesion should be avoided.
Internal root resorption (IRR) is defined as the loss of dental hard tissue due to osteoclastic activity inside the root canal space. IRR is a rare phenomenon that can manifest by means of a slow or rapid progression. Chronic pulpal inflammation and loss of predentin after trauma are thought to be potential predisposing factors for IRR.1 IRR is usually asymptomatic and detected incidentally during routine radiographic examinations.2 IRR can appear as round or oval radiolucent enlargements of the root canal space with well-defined margins, and shifted radiographs do not change the positional relationship of the canal to the resorptive entity. Histological samples of IRR show multinucleated giant cells in Howship's lacunae within the resorbed aspect of the root. If the IRR defect reaches the external root surface and perforates the root, destructive changes in periodontal ligament (PDL) and bone may result.1,3
Endodontic treatment should be initiated as soon as possible once IRR is detected. However, irregularities in the root canal space often make thorough cleaning, shaping, and obturation of the canal extremely difficult.1 If an IRR defect perforates a root, endodontic treatment should be followed by hermetic seal of the perforation site with a biocompatible sealing material.3
Favorable treatment outcomes for repair of perforated IRR with mineral trioxide aggregate (MTA) have been reported using both surgical and nonsurgical approaches.3,4,5 MTA has the ability to set in the presence of blood and/or moisture, is biocompatible, and has favorable sealing properties.6 In addition, MTA has the ability to stimulate cementogenesis and osteogenesis when used as a root-end filling or perforation repair material and placed in contact with periradicular and periodontal tissues.7,8
Calcium enriched mixture (CEM) cement is a tooth-colored calcium-silicate-based cement with the ability to set in the presence of moisture.9 Despite a chemical composition different from that of MTA, it's low cytotoxicity is comparable to MTA as shown in ex vivo and in vivo studies.9,10,11 In addition, the excellent biocompatibility of CEM cement has been well documented in animal and human studies.12,13,14 As an obturation material, CEM cement has also shown promising long-term sealing abilities.15,16
This case report describes the successful surgical management using CEM cement, of a failed orthograde MTA repair of a perforated internal root resorptive defect.
A 28-year old male with a non-significant medical history was referred to the dental school due to pain and discomfort associated with his maxillary right lateral incisor. He reported that the tooth had been endodontically treated four years ago. His dental records detailed a history of impact trauma and pulp necrosis, and previous radiographs showed a perforated IRR defect that had been treated with ProRoot MTA. The radiographs showed extrusion of MTA into the periodontium (Figure 1a). There was no information available regarding the number of treatment visits or whether a calcium hydroxide dressing had been placed between appointments. Updated radiographs showed complete resolution of the extruded MTA, as well as incomplete incorporation of the MTA filling into the IRR defect. A lateral radiolucent lesion and an inadequate apical obturation was evident (Figure 1b). Clinical exam elicited sensitivity to palpation of the periapical tissues as well as sensitivity to percussion (Figure 1c). Slight discoloration was visible mesiogingivally (Figure 1c). The pulpal and periapical diagnoses were inadequate previous root canal treatment and symptomatic apical periodontitis, respectively.
The first treatment plan presented to the patient was extraction of the incisor with subsequent implant placement, but upon further discussion it became evident that the patient was committed to salvaging the tooth. Surgical endodontic treatment was then discussed and decided upon. The risks and benefits of the procedure were reviewed, and informed consent was obtained.
The patient was given a 400 mg tablet of Ibuprofen to take pre-operatively to prevent post treatment discomfort, and was asked to rinse with 0.2% chlorhexidine mouthwash before the start of the surgery. The teeth were anesthetized with 2% Lidocaine with 1 : 80,000 epinephrine (DarouPakhsh, Tehran, Iran) via local infiltration. A sulcular incision followed by a full thickness flap was made to allow for adequate access to the lesion. Granulation tissue was removed in its entirety and sent for histopathological examination (Figure 2a). After thorough curettage of the bony crypts, hemostasis was achieved using hemostatic cotton pellets (Racellet 3, Pascal Int. Inc., Bellevue, WA, USA). The apical part of the root canal was prepared and cleaned with a minipiezon ultrasonic retrotip, (Joya electronics, Tehran, Iran) (Figures 2b and 2c), and the intra-canal MTA coronal to the defect was partially removed using ultrasonic tips to ensure direct contact of the new filling material with dentin. CEM cement (BioniqueDent, Tehran, Iran) powder and liquid were mixed according to the manufacturer's instructions and placed into the apical part of the root canal as well as into the perforated resorptive root defect. After radiographic confirmation of the proper filling of the resorptive lacuna (Figure 2d), the flap was repositioned and sutured. The sutures were removed after one week.
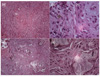
Histological exam demonstrated granulation tissue with an infiltration of chronic inflammatory cells (Figure 3a). Also visible were macrophages containing phagocytized particles, as well as a small number of giant cells (Figures 3b and 3c). Fragments of differently sized MTA particles were seen in select parts of the lesion (Figure 3d); and these fragments were generally encapsulated within fibrous connective tissue (Figure 3e).
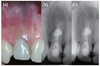
The patient returned for clinical and radiographic evaluation both one and two years post surgery. Clinical exams revealed that the lateral incisor was not sensitive to percussion or palpation, was functional, and had normal probing depths (- 2 mm). The overlying soft tissues were uninflamed and within normal limits (Figure 4a). Radiographic examination revealed that the lateral lesion once present had completely healed, and a healthy appearing PDL space was established (Figures 4b and 4c).
Key factors in the successful treatment of IRR are thorough cleaning of the resorptive lacuna and sealing of the defect with an appropriate biocompatible material.1 When IRR perforates root structure and forms a communication between the root canal space and the PDL, achieving the aforementioned goals of cleaning and sealing off the defect becomes more difficult and is sometimes impossible. If the clinician is unable to control the filling material by means of an orthograde approach, surgical intervention is then considered. However, repair of a perforation may be unachievable in certain cases where the perforation site is surgically inaccessible. Examples of such cases include palatal root perforations in maxillary anterior teeth, and interproximal perforations where root proximity with adjacent teeth becomes a concern. Three dimensional imaging using cone beam computed tomography (CBCT) is a useful tool that can illustrate the extent of the resorptive lesion, as well as depict the lesions' location and any perforation(s) which may not be distinguishable on two dimensional periapical radiographs.17 CBCT can also assist the clinician in establishing the most beneficial treatment plan for the patient. In the present case, the orthograde approach could not adequately address the goals of treatment, and thus insufficient obturation of the resorptive lacuna and defective sealing of the perforation resulted. In addition, the orthograde approach to treatment of resorptive defects provided only a low chance of completely removing all resorptive tissues and of thoroughly disinfecting the root canal. Conversely, the surgical approach allowed the clinician to better visualize the entire defect, and to remove all granulation tissues completely. Surgical treatment of resorptive defects also enabled the provider to control bleeding before obturation, to clean the apical third of the root canal with ultrasonic tips, and ultimately to seal off the root canal and all resorptive lacuna.
Previous research has shown that treatment outcomes following extrusion of MTA into periapical lesions are unpredictable. Perhaps most relevant is the point that MTA may not set after extrusion into periapical lesions.18 Environmental factors have been shown to affect the setting process and physical properties of MTA. For example, the sealing ability, push-out bond strength and surface hardness of MTA decreases significantly in acidic environments.19,20,21 When MTA extrudes into an inflammatory lesion it may contact pus, blood or exudates. The pH of pus collected from periapical lesions is acidic.22 Furthermore, serum and blood contamination during setting decreases the compressive strength and surface hardness of MTA, and changes its superficial microstructure.23,24 Since extruded MTA was not detected during the surgery, it is likely that the MTA never set after placement.
MTA is an osteoconductive biomaterial which up-regulates the expression of bone markers such as alkaline phosphatase, which is an important enzyme needed for bone formation.25 Set MTA is biocompatible and has very low cytotoxicity.6 Clinical reports have documented radiographically the resorption of extruded MTA, and the osseous healing of the lesion the MTA was extruded into.18,26 One of these studies showed a correlation between extruded MTA and persistent endodontic disease;18 however, histological evidence for this association was not available. Histological outcomes of the present case revealed the existence of phagocytosed microscopic particles of MTA by multinucleated giant cells throughout the lesion. Nevertheless, detailed chemical analysis was not done on these particles to confirm their chemical composition. Based on the patient's dental history and previous radiographs we assumed that these were MTA particles. This assumption needs further investigation. The composition of set MTA is different from that of unset MTA, and the tissue response to the latter is unpredictable. Although there is no information about the setting of extruded MTA in this case, the histological data shows an inflammatory reaction of the tissues to MTA particles. It is worth noting that inadequate cleaning of the root canal space and the presence of persistent infection originating from the primary treatment may be another reason for the inflammatory infiltrate present in the lesion. Bacteria-specific staining of the histological sections could potentially reveal the absence or presence of bacteria in the lesion.
While histological examination of the tissues showed that there were MTA particles present in the lesion, there were no radio-opaque particles visible in the pre-operative radiograph. MTA is composed primarily of tricalcium and dicalcium silicate, which produces calcium-silicate-hydrate gel and calcium hydroxide on hydration.27 Bismuth oxide, the opacifier in MTA powder, is present both as unreactive filler particles and as part of the structure of calcium-silicate-hydrate following hydration of the material.27 It was assumed that the total radiographic disappearance of the extruded MTA was due to the complete absorption of hydrated MTA, including both the unreacted and the hydrated bismuth oxide.18 However, the histological data from the present case shows that MTA may be present in the tissue while it is not visible radiographically. In other words, bismuth oxide may have been removed by inflammatory cells faster than the MTA as a whole. Nevertheless, this assumption should be confirmed with an elemental analysis of the specimen in question, and this deserves further study. It is also possible that the MTA particles are too small to be detected radiographically. This finding also warrants further study.
Animal studies have documented PDL regeneration when CEM cement is being used as a root-end filling and perforation repair material.12,13 As clinical reports have shown, CEM cement can be used as a root filling material in immature teeth and also in teeth with apical root resorption.15,16 CEM cement is a bioactive material and produces hydroxyapatite crystals on surface contact with phosphate-buffered saline and distilled water.28 Because CEM cement contains an endogenous reservoir of phosphate ions9 it's bioactivity is not dependent on exogenous phosphate. In comparison, the bioactivity of MTA has been shown to be dependent on environmental phosphate. Studies have revealed that phosphate containing solutions increase the biomineralization of MTA apical plugs and the sealing ability of MTA root-end fillings.29,30 Bioactivity of calcium-silicate-based cements has been correlated with their biocompatibility as well as sealing ability.31 The favorable biological outcome of the perforation repair in the present case is partly correlated with the bioactivity of CEM cement.
Effective root canal treatment of teeth afflicted with IRR and root perforations can prove to be a challenge; selection of the appropriate approach to treatment of these cases is essential for successful treatment outcomes. While MTA is a well-known and often used sealing material in perforations, extrusion of MTA into periradicular lesions should be avoided.
Figures and Tables
Figure 1
(a) Immediate postoperative periapical x-ray of orthograde MTA perforation repair (- 4 years before the surgical retreatment), extruded MTA was evident; (b) Preoperative periapical x-ray of the lateral incisor tooth shows complete resorption of extruded MTA and a lateral lesion adjacent to perforation site after 4 years; (c) Clinical photograph shows localized inflammation and swelling.
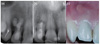
Figure 2
(a) Intra-radicular and bony defect after curettage of the lesion; (b) Using an ultrasonic retrotip in a coronoapical direction the apical portion of root canal was instrumented; (c) The canal was shaped and cleaned; (d) Filling and sealing of the apical portion of the root canal as well as the intra-radicular defect using calcium enriched mixture (CEM) cement was verified radiographically.

Figure 3
(a) Photomicrograph shows granulation tissue with chronic inflammatory cells (H&E staining, Mag. ×200); (b) A macrophage containing phagocyte particles (H&E staining, Mag. ×1,000); (c) A multinucleated giant cell (H&E staining, Mag. ×1,000); (d) Fragments of MTA of different sizes were seen (H&E staining, Mag. ×200); (e) A higher magnification of an MTA fragment which is encapsulated within fibrous connective tissue (H&E staining, Mag. ×1,000).
Acknowledgement
The authors gratefully acknowledge Dr. Arabella Christian from the Post-Graduate Endodontic Department, School of Dentistry, University of Maryland in Baltimore for her English revision of this manuscript.
References
1. Patel S, Ricucci D, Durak C, Tay F. Internal root resorption: a review. J Endod. 2010; 36:1107–1121.

2. Goultschin J, Nitzan D, Azaz B. Root resorption. Review and discussion. Oral Surg Oral Med Oral Pathol. 1982; 54:586–590.
3. Nunes E, Silveira FF, Soares JA, Duarte MA, Soares SM. Treatment of perforating internal root resorption with MTA: a case report. J Oral Sci. 2012; 54:127–131.

4. Hsien HC, Cheng YA, Lee YL, Lan WH, Lin CP. Repair of perforating internal resorption with mineral trioxide aggregate: a case report. J Endod. 2003; 29:538–539.

5. Altundasar E, Demir B. Management of a perforating internal resorptive defect with mineral trioxide aggregate: a case report. J Endod. 2009; 35:1441–1444.

6. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review-part II: leakage and biocompatibility investigations. J Endod. 2010; 36:190–202.

7. Noetzel J, Ozer K, Reisshauer BH, Anil A, Rössler R, Neumann K, Kielbassa AM. Tissue responses to an experimental calcium phosphate cement and mineral trioxide aggregate as materials for furcation perforation repair: a histological study in dogs. Clin Oral Investig. 2006; 10:77–83.

8. Regan JD, Gutmann JL, Witherspoon DE. Comparison of Diaket and MTA when used as root-end filling materials to support regeneration of the periradicular tissues. Int Endod J. 2002; 35:840–847.

9. Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J, Kheirieh S, Brink F. Comparison of mineral trioxide aggregate's composition with Portland cements and a new endodontic cement. J Endod. 2009; 35:243–250.

10. Mozayeni MA, Milani AS, Marvasti LA, Asgary S. Cytotoxicity of calcium enriched mixture cement compared with mineral trioxide aggregate and intermediate restorative material. Aust Endod J. 2012; 38:70–75.

11. Parirokh M, Mirsoltani B, Raoof M, Tabrizchi H, Haghdoost AA. Comparative study of subcutaneous tissue responses to a novel root-end filling material and white and grey mineral trioxide aggregate. Int Endod J. 2011; 44:283–289.

12. Samiee M, Eghbal MJ, Parirokh M, Abbas FM, Asgary S. Repair of furcal perforation using a new endodontic cement. Clin Oral Investig. 2010; 14:653–658.

13. Asgary S, Eghbal MJ, Ehsani S. Periradicular regeneration after endodontic surgery with calcium-enriched mixture cement in dogs. J Endod. 2010; 36:837–841.

14. Nosrat A, Peimani A, Asgary S. A preliminary report on histological outcome of pulpotomy with endodontic biomaterials vs calcium hydroxide. Restor Dent Endod. 2013; 38:227–233.

15. Nosrat A, Asgary S, Eghbal MJ, Ghoddusi J, Bayat-Movahed S. Calcium-enriched mixture cement as artificial apical barrier: a case series. J Conserv Dent. 2011; 14:427–431.

16. Asgary S, Nosrat A, Seifi A. Management of inflammatory external root resorption by using calcium-enriched mixture cement: a case report. J Endod. 2011; 37:411–413.

17. Ball RL, Barbizam JV, Cohenca N. Intraoperative endodontic applications of cone-beam computed tomography. J Endod. 2013; 39:548–557.

18. Nosrat A, Nekoofar MH, Bolhari B, Dummer PM. Unintentional extrusion of mineral trioxide aggregate: a report of three cases. Int Endod J. 2012; 45:1165–1176.

19. Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Fatemi A, Shiezadeh V, Ranjkesh B. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J Endod. 2008; 34:1226–1229.

20. Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod. 2010; 36:871–874.

21. Namazikhah MS, Nekoofar MH, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, Mohammadi MM, Dummer PM. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J. 2008; 41:108–116.

22. Nekoofar MH, Namazikhah MS, Sheykhrezae MS, Mohammadi MM, Kazemi A, Aseeley Z, Dummer PM. pH of pus collected from periapical abscesses. Int Endod J. 2009; 42:534–538.

23. Oloomi K, Saberi E, Mokhtari H, Mokhtari Zonouzi HR, Nosrat A, Nekoofar MH, Dummer PM. Evaluation of the effect of blood contamination on the compressive strength of MTA modified with hydration accelerators. Restor Dent Endod. 2013; 38:128–133.

24. Nekoofar MH, Oloomi K, Sheykhrezae MS, Tabor R, Stone DF, Dummer PM. An evaluation of the effect of blood and human serum on the surface microhardness and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010; 43:849–858.

25. Chen CL, Huang TH, Ding SJ, Shie MY, Kao CT. Comparison of calcium and silicate cement and mineral trioxide aggregate biologic effects and bone markers expression in MG63 cells. J Endod. 2009; 35:682–685.

26. Asgary S, Ehsani S. MTA resorption and periradicular healing in an open-apex incisor: a case report. Saudi Dent J. 2012; 24:55–59.

27. Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008; 41:408–417.

28. Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2009; 35:147–152.

29. Reyes-Carmona JF, Felippe MS, Felippe WT. A phosphate-buffered saline intracanal dressing improves the biomineralization ability of mineral trioxide aggregate apical plugs. J Endod. 2010; 36:1648–1652.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download