Abstract
Objectives
To evaluate the inhibitory effect of ursolic acid (UA)-containing composites on Streptococcus mutans (S. mutans) biofilm.
Materials and Methods
Composite resins with five different concentrations (0.04, 0.1, 0.2, 0.5, and 1.0 wt%) of UA (U6753, Sigma Aldrich) were prepared, and their flexural strengths were measured according to ISO 4049. To evaluate the effect of carbohydrate source on biofilm formation, either glucose or sucrose was used as a nutrient source, and to investigate the effect of saliva treatment, the specimen were treated with either unstimulated whole saliva or phosphate-buffered saline (PBS). For biofilm assay, composite disks were transferred to S. mutans suspension and incubated for 24 hr. Afterwards, the specimens were rinsed with PBS and sonicated. The colony forming units (CFU) of the disrupted biofilm cultures were enumerated. For growth inhibition test, the composites were placed on a polystyrene well cluster, and S. mutans suspension was inoculated. The optical density at 600 nm (OD600) was recorded by Infinite F200 pro apparatus (TECAN). One-way ANOVA and two-way ANOVA followed by Bonferroni correction were used for the data analyses.
Results
The flexural strength values did not show significant difference at any concentration (p > 0.01). In biofilm assay, the CFU score decreased as the concentration of UA increased. The influence of saliva pretreatment was conflicting. The sucrose groups exhibited higher CFU score than glucose group (p < 0.05). In bacterial growth inhibition test, all experimental groups containing UA resulted in complete inhibition.
Composite resin has gained wide popularity in dental practice in the past few decades.1 The basic properties, such as mechanical, physical and bonding properties have been greatly improved as a result of numerous investigations. However, the recurrent caries is the most common cause of failure in composite restorations.2 To overcome this problem, many researchers have been trying to develop an antibacterial restoration. There have been numerous attempts including addition of sliver nanoparticles, quaternary ammonium polyethylenimine nanoparticles, antibiotics, chlorhexidine diacetate, alkylated ammonium chloride derivatives, and methacryloyloxydodecyl pyridinium bromide.3-6 However, they had several disadvantages, such as discoloration of the material, decreased physical property and gradual diminution of antibacterial effect.7,8 Thus, no commercially successful product has been developed at the present time.
Even though derivatives from natural products have been a major source of therapeutic agents in medicine, they have received minimal attention in the field of dentistry. Triterpenoids are well known for its anti-cancer, anti-wrinkle, and muscle growth effect, and it also has an antibacterial effect.9 As they are natural derivatives, triterpenoids do not have pronounced toxic effect on normal cells.10 Ursolic acid (UA, Figure 1) is one of those triterpenoids which possess antibacterial effects reported by a number of researchers.11
Streptococcus mutans (S. mutans) is the bacteria which is involved in the transition from nonpathogenic to cariogenic biofilms, although many other microorganisms also take a role in the pathogenesis of the dental caries.12 Therefore, antibacterial agent which is effective against S. mutans might be considered as an anticariogenic agent.
When conducting an in vitro experiment on the antibacterial activity of the restorative material, the environment must be controlled in such a way that is physiologically similar to the oral cavity. Since the previous literature had proven that there is a clear difference in the bacterial expression between biofilm and planktonic state, a biofilm model should be considered essential.13 Many biofilm models have been proposed to evaluate the antibacterial activity of modified composite resin.14 However, the consensus over the accurate method has not been established yet. This controversy is due to the complexity of controlling the environment, such as the method of biofilm formation, carbohydrate source, period of incubation, replenishment of the medium, and so on. The objective of this study was to evaluate the inhibitory effect of the composite resins containing UA on S. mutans biofilm in vitro.
Composite resins with different concentrations of UA (U6753, Sigma Aldrich, St. Louis, MO, USA) were prepared. The UA was dissolved in 10 mL of acetone in a glass bowl, and then commercial nanofilled composite (Filtek Z350 A2 shade, 3M ESPE, St Paul, MN, USA) was dissolved and stirred thoroughly with spatula. Afterwards, the glass bowl was covered with aluminum foil and stored in room temperature for 24 hours for evaporation of residual acetone. The mixtures were stirred every 6 hours to facilitate the evaporation of acetone. The composite resin mixed only with acetone served as a negative control (0%).
When the organic molecules are added to composite, the physical properties are expected to be decreased. Flexural strength test was intended to rule out the detrimental effect of UA on physical property of composite resin. Five different concentrations of 0.04, 0.1, 0.2, 0.5, and 1 wt% were used for analysis of flexural strength. Rectangular bar-shaped composite resin specimens with dimension of 2 × 2 × 25 mm were prepared based on the ISO 4049 and stored in saline at room temperature for 24 hours. A total of 6 specimens were prepared for each experimental concentrations of UA. Each specimen was mounted to Instron 5942 (Illinois Tool Works Inc., Norwood, MA, USA), and 3-point bending force was applied at a crosshead speed of 1 mm/min until the specimen fractured. The maximum stress was recorded directly from the testing machine and verified by the Bluehill software, version 2.25 (Illinois Tool Works Inc.).
Three experimental concentrations of UA (0.1, 0.2 and 0.5 wt%) and a control group (0%) were selected for further analysis. For different saliva treatments, the composite resin specimens were submerged in either phosphate-buffered saline (PBS, pH = 7.2, non-coating group) or unstimulated whole saliva (UWS, saliva coating group) and placed on a rocking incubator for 2 hours for formation of biofilm. For nutrient source provided for bacterial growth, either glucose or sucrose was added to medium. The combinations of all these variables are as displayed in Table 1.
UWS was collected from four healthy volunteers by the spitting method. All participants had undergone dental examination prior to the experiment to ensure that they were free from any acute caries or periodontal disease. Saliva was collected between 9:00 a.m. to 11:00 a.m. to minimize the effects of diurnal variability on salivary composition.15 The collected samples were centrifuged at 3,500 g for 10 minutes to remove any cellular debris. The resulting supernatant was then filter-sterilized through a Stericup & Steritop (Millipore, Billerica, MA, USA), and stored in 4℃ before use. S. mutans UA159 was grown in the brain heart infusion (BHI) agar plate. A colony of S. mutans was transferred to BHI broth, and the broth was incubated overnight. The culture was then re-suspended to BHI broth in 1 : 20 ratio and incubated again until it reached exponential phase. The optical density at 600 nm (OD600) was measured, and the broth was used when the OD600 reached 0.5 (approximately 6.5 × 107 CFU per mL).
The experimental composite resin was filled in Teflon mold with a cylindrical cavity (5 mm diameter × 2 mm height) with two glass slides on both sides and light cured for 40 seconds. Since biofilm formed on the upper surface, careful attention was required not to turn the specimen upside down. The bottom side was marked with an oil-based pen for identification. After polishing the margin, they were sterilized by ethylene oxide (EO) gas. Surface irregularity may have occurred during preparation of experimental composite resin by impregnation of air or inhomogeneous composition. To compare the surface constellation, a random sample was chosen prior to biofilm assay and investigated with confocal laser scanning microscopy (CLSM, Axiovert 200M, Carl Zeiss, Thornwood, NY, USA). Since the ability to generate biofilm by this experimental method had already been verified by the pilot experiment, CLSM was not taken separately to confirm the biofilm formation.
The sterilized composite resin disks were transferred to a polystyrene 48-well (flat-bottom) cell culture clusters (Corning Inc., Corning, NY, USA). The prepared S. mutans suspension was diluted with BHI broth which was kept warm in incubator. The medium contained 20 mM of either glucose or sucrose as a carbohydrate source.
The composite resin disks which were treated with either UWS or PBS were inoculated with those medium containing 1 : 100 dilution of S. mutans suspension. The plate was transferred to incubator and kept at 37℃ in a 5% CO2 for 24 hours. Afterwards, the specimens were rinsed twice with 2 mL of PBS to remove planktonic cells. The specimens were then placed in a glass tube with 3 mL of PBS and sonicated for 30 seconds using pulse at 25 W three times with simultaneous cooling by placing the tube in ice. The disrupted biofilm suspension was diluted serially and plated in duplicate on BHI agar. The plating was carried out by automatic sample plater (easySpiral, Interscience, Saint Nom, France). After 48 hours, CFUs were counted visually, scaled by dilution factors. If CFU value between duplicates differed more than 20%, the data was discarded. For statistical reason, all data acquired on the same day were also discarded. The whole procedure of the biofilm assay was summarized in Figure 2.
A small amount of composite resin was molded to a crescent-shape at the bottom of polystyrene 96-well (flat-bottom) cell culture clusters (Corning Inc., Corning, NY, USA). An impression of the well with the specimen was taken using a vinyl polysiloxane impression material. A metal mold imitating the impression was fabricated in the laboratory. An appropriate amount of the experimental resin was pressed into the mold and then introduced to the well and light cured. A total of 5 specimens were fabricated for each experimental group. The cell culture plate was sterilized by EO gas.
The specimen in the well was treated with 200 µL of either PBS or UWS for 2 hours. In the well assigned for positive/negative controls, no specimen was placed and was not treated with PBS or UWS. Trypton-vitamin (TV) medium supplemented with 0.5% glucose or sucrose was inoculated with 1 : 100 dilutions of S. mutans broth. TV medium (either with glucose or sucrose) without S. mutans was placed in a well for negative control, and the same medium with S. mutans was used for positive control. To maintain anaerobic condition and prevent the evaporation of TV media, sterile mineral oil (50 µL per well) was placed on each well at the top of the TV medium. The plate was placed in Infinite F200 pro (TECAN, Salzburg, Austria) and incubated at 37℃. The OD600 of the TV medium in the each well was recorded at every 30 minutes for 24 hours. The initial data analysis was carried out with Magellan 7 software (TECAN group Ltd., Mannedorf, Switzerland), and the change in OD600 was calculated with Microsoft Excel (Microsoft Corp., Redmond, WA, USA). If a well was dehydrated due to insufficient protection, the data from the well was excluded from the analysis. The procedure was performed in duplicate.
One-way ANOVA was used to evaluate the results of the flexural strength test. Two-way ANOVA followed by the Bonferroni correction was used for biofilm assay to analyze statistical significance. Statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). The adjusted p value less than 0.05 was considered to be statistically significant.
The flexural strength of each group is shown in Figure 3. All values were above 80 MPa, which was higher than the minimum requirement of ISO standard. The flexural strength did not show statistically significant difference at any concentration (p > 0.05). However, there was a tendency of decreasing flexural strength as the concentration of UA increased.
The results of the CFU values of S. mutans in biofilm are represented in Table 2. The two-way ANOVA demonstrated that CFU scores of S. mutans were significantly influenced by both concentration of UA and pretreatment condition in both groups. There was no interaction between concentration of UA and salivary pretreatment condition in glucose groups. In contrast, there was an interaction when sucrose was given as the carbohydrate source. In glucose groups, the CFU scores of S. mutans were significantly influenced by both concentration of UA and salivary pretreatment.
As depicted in Figure 4, the CFU score decreased as the concentration of UA increased. When glucose was offered as the carbohydrate source, significant decrease was shown among 0, 0.1, and 0.2%, regardless of the salivary pretreatment condition. There was no significant difference between 0.2% and 0.5%. However, when sucrose was given, statistically significant difference was only found between 0 and 0.5% in the saliva coating group. The influence of saliva pretreatment was conflicting. When glucose was given, significantly lower CFU score in coating group was found only in lower concentrations (0% and 0.1%). On the contrary, when sucrose was given as a nutrient, only 0.1 and 0.5% concentration demonstrated statistical significance. As far as the carbohydrate sources are concerned, the sucrose groups exhibited higher CFU values than those of glucose groups.
Addition of UA resulted in a complete inhibition of growth when TV-glucose medium was given. In TV-sucrose medium, two groups (0.1 and 0.2% without saliva coating) showed initial lag phase. After this inhibition phase, of the uninhibited control. In addition, those groups entered stationary phase at a substantially lower optical density as compared to the control culture. All other groups with TV-sucrose showed complete inhibition of growth throughout the observation period (Figure 5).
There are many other compounds that are reported to have an antimicrobial effect against cariogenic bacteria.16,17 Among those, UA was selected in this experiment for several reasons. First of all, UA exhibited inhibition of S. mutans at a very low concentration (MIC90 = 2 µg/mL) in planktonic condition.18 In addition, due to the hydrophobic nature of the reagent, it can be blended with the composite resin matrix and is not easily eluted in the saliva. Since the flexural strength was not statistically significantly affected by addition of the UA, 0.1, 0.2, and 0.5 wt% were selected for the analysis of antibacterial effect in present study.
The concentration of UA used in present experiment was much higher than the MIC value identified by a previous study because the action of reagent was speculated to be limited by cured resin matrix.18 As demonstrated in both analyses, the growth of S. mutans seems to be disturbed by the addition of UA. How UA suppresses the bacterial growth of S. mutans is not fully understood yet. According to Cowan, antibacterial compounds aim at bacterial eradication via one or more of the following modalities: (i) disruption of cell wall and/or cell membrane, interacting with surface-adsorbed components, (ii) inhibition of protein synthesis or nucleic acid metabolism, and (iii) inhibition of enzyme activity through oxidation.6,19 One study examined the effect of UA in Listeria monocytogenes species. The results showed inhibited peptidoglycan turnover and reduced profile of muropeptides obtained after digestion of peptidoglycan with mutanolysin, suggesting that peptidoglycan metabolism is a cellular target of UAs.20 Another study demonstrated that the plant extract from Zizyphi Fructus had inhibitory activity against insoluble glucan formation by glucosyltransferase from S. mutans.21 One of the isolated compounds was found to be UA. Glucosyltransferases (GtfB, GtfC, GtfD) plays a crucial role in bacterial attachment, and their products were thought to act as diffusion-limiting macromolecules in plaque.22
S. mutans is known to express different growth patterns depending on the carbohydrate source. Therefore, both glucose and sucrose were tested in this experiment. S. mutans can attach initially to saliva coated surfaces through sucrose-independent mechanisms.23 However, this bacterium binds to the glucan-coated surfaces, in larger numbers and with higher adhesion strength than to uncoated or saliva-coated apatitic surfaces.24,25 Thus the greater number of CFU value in the groups to which sucrose was given as carbohydrate source wasis somewhat expected.
The influence of saliva pretreatment was conflicting as it had no effect in some conditions, while it resulted in decreased CFU value in the other conditions. The pretreatment with UWS was intended to form biofilm prior to bacterial inoculation, and biofilm is known to play a crucial role in initial attachment and subsequent growth of the bacteria. The UA might have reduced the effect of pre-formed biofilm in coating group. One study showed inhibition of bacterial colonization when Escherichia coli was added to a 24-hour biofilm with UA.26
Bacterial growth curve showed that UA interfered with the growth of S. mutans. However, the pattern of inhibition did not correspond with the result of biofilm assay, which displayed obvious differences in many parameters. A Possible reason for the difference is the measurement method, such as growth medium, air condition, and size of the well. Since there is a continuous exchange of fluid in the oral environment, the size of 96-well plate, which resulted in smaller amount of medium, might have possessed limited clinical relevance. When comparison is made between optical density of positive control without specimen and control groups with specimen at stationary phase, the positive control reached higher value. Since this experiment was intended to verify the possibility of UA as an anti-cariogenic agent, the reagent was simply mixed into the composite resin with a solvent. As a result, the antibacterial effect of the acetone might have had an impact.
There are several limitations in present study. When bacterial biofilm is formed on the surface of the teeth, it is produced by the interaction between bacteria, rather than by a single bacterium. In addition, it is well known that the composition of bacteria in dental plaque changes over time. Since this experiment was carried out only with S. mutans, which is involved in the initial process of dental caries, the effect of UA after initial bacterial colonization would be beyond the scope of this study. Even though every effort was made to maintain uniform procedure, considerable variation in the data occurred. This might be due to the condition of the bacteria, the surface quality of each specimen, or the error generated from the procedure of sonication and serial dilution. Before clinical application, further studies are required to determine the optimum concentration and the addition method of UA.
Antimicrobial tests showed that UA, when incorporated in dental composite resins at low concentration (0.1 - 0.5 wt%), exhibited an antibacterial effect against S. mutans for the experimental periods. In the limitation of this experimental conditions, UA included in the composite showed inhibitory effect on biofilm formation and growth of S. mutans.
Figures and Tables
Figure 1
Structure of the ursolic acid [3β-hydroxy-urs-12-en-28-oic acid; C30H48O3; molecular weight 456.71].
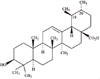
Figure 2
Procedures of biofilm assay.
UA, ursolic acid; PBS, phosphate buffered saline; UWS, unstimulated whole saliva; BM, BHI (brain heart infusion) media; S. mutans, Streptococcus mutans.

Figure 3
Flexural strength of composite resin with various concentrations of ursolic acid. The error bars represent standard deviations (n = 6). There was no statistically significant difference at any concentration (p > 0.05).
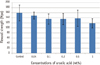
Figure 4
Change in the colony forming unit of Streptococcus mutans as the concentration of ursolic acid (UA) increases. (a) Glucose groups; (b) Sucrose groups. In glucose groups, the colony forming unit (CFU) scores were influenced by both UA concentration and saliva treatment (p < 0.05). In sucrose groups, there was a significant difference between 0 and 0.5% (p < 0.05).

Notes
References
1. Haj-Ali R, Walker MP, Williams K. Survey of general dentists regarding posterior restorations, selection criteria, and associated clinical problems. Gen Dent. 2005; 53:369–375.
2. Opdam NJ, Bronkhorst EM, Roeters JM, Loomans BA. A retrospective clinical study on longevity of posterior composite and amalgam restorations. Dent Mater. 2007; 23:2–8.

3. Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006; 27:3995–4002.

4. Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young AM. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005; 26:7145–7153.

5. Stobie N, Duffy B, McCormack DE, Colreavy J, Hidalgo M, McHale P, Hinder SJ. Prevention of Staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol-gel coating. Biomaterials. 2008; 29:963–969.

6. Imazato S, Kinomoto Y, Tarumi H, Torii M, Russell RR, McCabe JF. Incorporation of antibacterial monomer MDPB into dentin primer. J Dent Res. 1997; 76:768–772.

7. Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K. Development of an antimicrobial resin-a pilot study. Dent Mater. 2011; 27:322–328.

8. Nohr RS, Macdonald JG. New biomaterials through surface segregation phenomenon: new quaternary ammonium compounds as antibacterial agents. J Biomater Sci Polym Ed. 1994; 5:607–619.

9. Liu J. Oleanolic acid and ursolic acid: research perspectives. J Ethnopharmacol. 2005; 100:92–94.

11. Fontanay S, Grare M, Mayer J, Finance C, Duval RE. Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J Ethnopharmacol. 2008; 120:272–276.

12. Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011; 90:294–303.
13. Costerton JW, Lewandowski Z, DeBeer D, Caldwell D, Korber D, James G. Biofilms, the customized microniche. J Bacteriol. 1994; 176:2137–2142.

14. Shapiro S, Giertsen E, Guggenheim B. An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouthrinses. Caries Res. 2002; 36:93–100.

15. Hardt M, Witkowska HE, Webb S, Thomas LR, Dixon SE, Hall SC, Fisher SJ. Assessing the effects of diurnal variation on the composition of human parotid saliva: quantitative analysis of native peptides using iTRAQ reagents. Anal Chem. 2005; 77:4947–4954.

16. Allaker RP, Douglas CW. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents. 2009; 33:8–13.

17. Jeon JG, Rosalen PL, Falsetta ML, Koo H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res. 2011; 45:243–263.

18. Kim MJ, Kim CS, Park JY, Lim YK, Park SN, Ahn SJ, Jin DC, Kim TH, Kook JK. Antimicrobial effects of ursolic acid against mutans streptococci isolated from Koreans. Int J Oral Biol. 2011; 36:7–11.
20. Kurek A, Grudniak AM, Szwed M, Klicka A, Samluk L, Wolska KI, Janiszowska W, Popowska M. Oleanolic acid and ursolic acid affect peptidoglycan metabolism in Listeria monocytogenes. Antonie Van Leeuwenhoek. 2010; 97:61–68.

21. Kohda H, Kozai K, Nagasaka N, Miyake Y, Suginaka H, Hidaka K, Yamasaki K. Prevention of dental caries by Oriental folk medicines-active principles of Zizyphi Fructus for inhibition of insoluble glucan formation by cariogenic bacterium Streptococcus mutans. Planta Med. 1986; (2):119–120.
22. Ooshima T, Matsumura M, Hoshino T, Kawabata S, Sobue S, Fujiwara T. Contributions of three glycosyltransferases to sucrose-dependent adherence of Streptococcus mutans. J Dent Res. 2001; 80:1672–1677.

23. Gibbons RJ. Role of adhesion in microbial colonization of host tissues: a contribution of oral microbiology. J Dent Res. 1996; 75:866–870.

24. Cross SE, Kreth J, Zhu L, Sullivan R, Shi W, Qi F, Gimzewski JK. Nanomechanical properties of glucans and associated cell-surface adhesion of Streptococcus mutans probed by atomic force microscopy under in situ conditions. Microbiology. 2007; 153:3124–3132.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download