Abstract
Objectives
Fast-setting pozzolan cement (Endocem, Maruchi) was recently developed. The aim of this study was to investigate the effects of various root canal irrigants on the washout of Endocem in comparison to the previously marketed mineral trioxide aggregate (ProRoot; Dentsply) in a furcal perforation model.
Materials and Methods
ProRoot and Endocem were placed into acrylic molds on moist Oasis. Each mold was then immediately exposed to either physiologic saline, 2.5% sodium hypochlorite (NaOCl), or 2% chlorhexidine (CHX) under gentle shaking for five minutes. Washout testing was performed by scoring scanning electron microscope (SEM) images.
Furcal perforation may occur as the consequence of a procedural error or occasionally as the result of a pathologic process such as dental caries and root resorption.1 Mineral trioxide aggregate (MTA) is a promising material that has been widely used in furcal perforation repair because of its excellent biocompatibility, superior sealing, and setting ability even in the presence of blood.2,3 However, some shortcomings of MTA have also been reported, including its long setting time.4 If a furcal perforation is repaired in this fashion, the clinician should apply wet cotton to the MTA and make another appointment for further treatment to provide the MTA the time to be set. Otherwise, "washout" of the unset MTA could occur during use of irrigation solution.
Recently, MTA-derived pozzolan cement (Endocem, Maruchi, Wonju, Korea) was introduced in the endodontic market. Endocem sets quickly without the addition of a chemical accelerator. Instead, it contains small particles of pozzolan cement to increases the surface contact with the mixing liquid and provides rapid setting. Recently, Choi et al. showed that Endocem set much quicker and was more resistant to "washout" mediated by fetal bovine serum than the previously marketed MTA (ProRoot, Dentsply, Tulsa, OK, USA) in a root-end filling model.5 However, there is little information about the effects of various root canal irrigants including physiologic saline, sodium hypochlorite (NaOCl), and chlorhexidine (CHX). Therefore, the aim of this study was to investigate the effects of various root canal irrigants on "washout" of the MTA-derived pozzolan cement in a furcal perforation model.
ProRoot and Endocem were mixed according to the manufacturers' instructions. After mixing, each material was placed into a 1 mm × 2 mm acrylic mold on saline-moistened Oasis which simulated periodontal tissue in furcation area (n = 10) (Figure 1a). Each mold was immersed in either physiologic saline, 2.5% NaOCl, or 2% CHX immediately with gentle shaking (Compact Rocker-CR300, FINEPCR, Gunpo, Korea) for five minutes, and then was stored in an incubator at 95 ± 5% relative humidity and 37℃ for 24 hours. Scanning electron microscopy (SEM) was performed using a JSM-6360 system (JEOL, Tokyo, Japan) operated at 10 kV (Figures 2 and 3). The washout score was calibrated and evaluated according to the criteria listed in Table 1 by three dentists who had no knowledge of the source of the specimens.
The data were then analyzed by an independent samples t-test to compare the two materials (p = 0.05). The Kruskal-Wallis test was used to evaluate the effects of the three irrigants on washout score (p = 0.05).
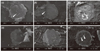
Washout scores were assigned to investigate whether the irrigation solutions affected the washout of ProRoot and Endocem. As shown in Figure 1b, ProRoot showed higher washout scores than Endocem under all irrigation solutions (p < 0.05). Furthermore, the washout scores of ProRoot and Endocem were significantly lower in the NaOCl-treated group compared to the saline- and CHX-treated groups (p < 0.05). Representative SEM images used for washout scoring are shown in Figure 2.
At the microstructure level, the crystal size of the NaOCl-treated group was greater than that of the saline- and CHX-treated groups in both ProRoot and Endocem samples. Notably, little crystalline formation and an amorphous layer were observed in the CHX-treated groups (Figure 3).
For desirable results of furcal perforation repair, the material should have several ideal characteristics including biocompatibility, good sealing properties, insolubility in tissue fluids, and dimensional stability.4,6 Among various materials, MTA has been recommended for the treatment of furcal perforations.7-9 Despite its many favorable properties, MTA has some drawbacks such as a long setting time, which is still problematic for clinical applications. The slow setting results in handling difficulties and washout of the MTA newly placed in the repaired area during the subsequent irrigation procedure.10,11
Many researchers have attempted to develop fast-setting MTA or its derivatives.11-16 However, most of these trials have focused on the addition of chemical setting accelerators, some of which proved to have detrimental physical and biologic effects.15,16 Endocem, a fast-setting MTA-derived pozzolan type of cement, was recently developed. Choi et al. demonstrated that Endocem had a much shorter setting time and more washout resistance under the presence of fetal bovine serum compared to ProRoot.5 They also suggested that the decreased setting time might be associated with the increase in the early strength of Endocem. Similarly, Endocem demonstrated a lower washout score than ProRoot under all irrigation solutions in the present study (p < 0.05).
We also observed that the washout scores of ProRoot and Endocem were significantly different depending on the irrigation solution used. The NaOCl-treated group showed significantly lower washout scores compared to the salineand CHX-treated groups for both ProRoot and Endocem (p < 0.05) (Figure 1b). There were some studies that showed NaOCl decreased the setting time and microleakage of MTA. Kogan et al. reported that different additives produced a wide range of MTA setting times, and NaOCl gel decreased the setting time while saline and CHX gel resulted in an increase in setting time.12 Furthermore, Uyanik et al. reported that the sealing of furcal perforation repaired with MTA was affected by the irrigation regimens, and the samples irrigated with NaOCl had the lowest leakage values.17 Notably, NaOCl has an alkaline pH of 9.0 - 10.5 whereas CHX and saline have neutral pH.18 The literature indicated that lower pH environments may affect various physical and chemical properties of MTA negatively.19-21
At the microstructure level, the CHX group demonstrated different crystal morphology than the other groups. Evaluation of the SEM images revealed a distinct lack of crystal structures in all groups exposed to CHX when compared to the saline and NaOCl groups (Figure 3). Recently, Hong et al. reported that CHX adversely affected the physical properties and hydration behaviors of MTA when it was in contact with MTA before initial setting.22 The authors explained that crystal structures were not apparent on the surface of the CHX-treated samples, and they were not the typical calcium hydroxide crystals on energy dispersive x-ray spectroscopic analysis. These findings may explain why the washout resistance of the CHX-treated group was lower than that of the NaOCl-treated group in this study.
Our results indicate that Endocem exhibited superior washout resistance compared to ProRoot, especially in the NaOCl-treated group. Thus, within the limitation of this study, Endocem may be considered a substitute for ProRoot in a single-visit scenario of conventional root canal treatment with furcal perforation repair because it is less loosened during the setting reaction. Furthermore, NaOCl can be considered a more suitable irrigant than physiologic saline and CHX in a tooth with furcal perforation repaired using Endocem in terms of washout resistance. However, further studies regarding the biocompatibility of Endocem with periodontal tissue should be considered.
Figures and Tables
Figure 1
(a) Schematic of the model used for the experiment; (b) Washout scores of ProRoot (PR) and Endocem (EC). Each point and bar represents the mean ± SD. The capital letters represent significant differences between ProRoot and Endocem for each root canal irrigant, and the lowercase letters represent significant differences between root canal irrigants for each repair material. SLN, physiologic saline; NaOCl, sodium hypochlorite; CHX, chlorhexidine.

Figure 2
SEM observation indicating the washout tendency of ProRoot (a - c) and Endocem (d - f) in the presence of various root canal irrigants (×30). SLN, physiologic saline (a and d); NaOCl, sodium hypochlorite (b and e); CHX, chlorhexidine (c and f). White arrowheads indicate the areas where wash-out happened.
Acknowledgements
This paper was supported by Fund of Biomedical Research Institute, Chonbuk National University Hospital in 2013 and Rising Support Project of Wonju Medical Industry Technovalley Foundation in 2012.
References
1. Pace R, Giuliani V, Pagavino G. Mineral trioxide aggregate as repair material for furcal perforation: case series. J Endod. 2008; 34:1130–1133.

2. Ferris DM, Baumgartner JC. Perforation repair comparing two types of mineral trioxide aggregate. J Endod. 2004; 30:422–424.

3. Shahi S, Rahimi S, Hasan M, Shiezadeh V, Abdolrahimi M. Sealing ability of mineral trioxide aggregate and Portland cement for furcal perforation repair: a protein leakage study. J Oral Sci. 2009; 51:601–606.

4. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part I: chemical, physical, and antibacterial properties. J Endod. 2010; 36:16–27.

5. Choi Y, Park SJ, Lee SH, Hwang YC, Yu MK, Min KS. Biological effects and washout resistance of a newly developed fast-setting pozzolan cement. J Endod. 2013; 39:467–472.

6. Hardy I, Liewehr FR, Joyce AP, Agee K, Pashley DH. Sealing ability of One-Up Bond and MTA with and without a secondary seal as furcation perforation repair materials. J Endod. 2004; 30:658–661.

7. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413.

8. Biswas M, Mazumdar D, Neyogi A. Non surgical perforation repair by mineral trioxide aggregate under dental operating microscope. J Conserv Dent. 2011; 14:83–85.

9. Unal GC, Maden M, Isidan T. Repair of furcal iatrogenic perforation with mineral trioxide aggregate: two years follow-up of two cases. Eur J Dent. 2010; 4:475–481.

10. Guneser MB, Akbulut MB, Eldeniz AU. Effect of various endodontic irrigants on the push-out bond strength of biodentine and conventional root perforation repair materials. J Endod. 2013; 39:380–384.

11. Wiltbank KB, Schwartz SA, Schindler WG. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and Portland cement. J Endod. 2007; 33:1235–1238.

12. Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006; 32:569–572.

13. Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009; 35:550–554.

14. AlAnezi AZ, Zhu Q, Wang YH, Safavi KE, Jiang J. Effect of selected accelerants on setting time and biocompatibility of mineral trioxide aggregate (MTA). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111:122–127.

15. Lee BN, Hwang YC, Jang JH, Chang HS, Hwang IN, Yang SY, Park YJ, Son HH, Oh WM. Improvement of the properties of mineral trioxide aggregate by mixing with hydration accelerators. J Endod. 2011; 37:1433–1436.

16. Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005; 38:834–842.

17. Uyanik MO, Nagas E, Sahin C, Dagli F, Cehreli ZC. Effects of different irrigation regimens on the sealing properties of repaired furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e91–e95.

18. Zehnder M, Kosicki D, Luder H, Sener B, Waltimo T. Tissue-dissolving capacity and antibacterial effect of buffered and unbuffered hypochlorite solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002; 94:756–762.

19. Saghiri MA, Shokouhinejad N, Lotfi M, Aminsobhani M, Saghiri AM. Push-out bond strength of mineral trioxide aggregate in the presence of alkaline pH. J Endod. 2010; 36:1856–1859.

20. Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod. 2010; 36:871–874.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download