Abstract
Objectives
The aim of this study was to determine the effect of epigallocatechin gallate (EGCG) on the shear bond strength of composite resin to bleached enamel.
Materials and Methods
Ninety enamel surfaces of maxillary incisors were randomly divided into 9 groups as follows: G1: control (no bleaching); G2: bleaching; G3: bleaching and storage for seven days; G4 - 6: bleaching and application of 600, 800 and 1,000 µmol of EGCG-containing solution for 10 minutes, respectively; G7 - 9: bleaching and application of 600, 800 and 1,000 µmol of EGCG-containing solution for 20 minutes, respectively. The specimens were bleached with 30% hydrogen peroxide gel and a composite resin cylinder was bonded on each specimen using a bonding agent. Shear bond strength of the samples were measured in MPa. Data was analyzed using the two-way ANOVA and Tukey HSD tests (α = 0.05).
Results
The maximum and minimum mean shear bond strength values were observed in G1 and G2, respectively. Time and concentration of EGCG showed no significant effects on bond strength of the groups (p > 0.05). Multiple comparison of groups did not reveal any significant differences between the groups except for G2 and all the other groups (p < 0.05).
One of the most conservative esthetic treatment procedures, requested by a large number of patients, is tooth bleaching. This procedure should be combined with toothcolored restorative procedures in most cases to achieve optimal esthetic results.1 Materials which cause tooth discoloration include organic materials with complex double bonds.2 In the majority of bleaching procedures, some forms or derivatives of hydrogen peroxide with various concentrations and different techniques are used.3,4 It has been demonstrated that bleaching depends on an oxidation reaction, in which oxygen free radicals diffuse into enamel and dentin structures due to their low molecular weight. It has been reported that the bleaching agent reacts with pigmented materials, resulting in the opening of pigmented carbon rings and converting the rings to smaller and more light-colored intermediary chains. These processes cause the teeth to appear brighter.5,6
In all the bleaching procedures, when composite resin is placed on bleached enamel and dentin surfaces, there is a short period of decrease in bond strength to composite resin, which is attributed to remnants of oxygen or peroxide in tooth structure, preventing polymerization of the resin bonding agent and formation of sufficient resin tags in the etched enamel.
One of the most common techniques to restore bond strength after bleaching procedures is to delay the bonding procedure after bleaching.7 Another technique to avoid such a delay and enable immediate bonding after bleaching procedures is to apply antioxidative agents. Several studies have shown that use of 10% sodium ascorbate and its salts after bleaching procedures restores bond strength to bleaching enamel up to a level achieved without the use of bleaching agents.8-11 Other antioxidants include butylhydroxyanisole, catalase, glutathion peroxidase, sodium bicarbonate and alpha-tocopherol.12,13
Polyphenol compounds of green tea are antioxidants used in modern dentistry. Green tea which is extracted from the camellia plant contains several catechin compounds. Catechins are chemical antioxidants, which have the capacity to destroy free radicals. Epigallocatechin gallate (EGCG) is the most active and abundant catechin in green tea. It is soluble in water and is a safe material. It has a number of useful properties, including treatment of cardiac diseases, cancers and obesity.14 Recent studies have shown that green tea has a great potential to prevent various diseases in man. It can inhibit tumors in their early stages and prevent their progress. It also prevents cancer and increases resistance of lipoproteins to oxidation outside of the living body. Various studies have shown that catechins in green tea are rapidly metabolized, which is attributed to their antioxidative activity.5,14,15 These polyphenols are used in dentistry to prevent loss of alveolar bone in periodontal diseases, to reduce dentin loss in erosion and abrasion and to prevent caries.16-18 The mechanism has been suggested to be due to the inhibitory effect of polyphenol metalloproteinases. Therefore, the present study was carried out to evaluate the effect of EGCG in green tea on the shear bond strength of composite resin to bleached enamel. The null hypothesis of this study was EGCG in green tea does not influence the shear bond strength of composite resin to bleached enamel.
In order to prepare different concentrations of EGCG solutions, 95% extract of EGCG (SIGMA-Aldrich Co., St. Louis, MO, USA) was used; 600-, 800- and 1000-µmol concentrations were prepared by diluting the extract in water. The pH value of the diluted solutions (pH) was 6.25 - 6.40.
In this study, 90 maxillary incisors were used. All the teeth were obtained in a period of 3 months in private dental offices and clinics of Hamadan, after informed written consent was obtained under a protocol approved by the Ethics Committee of the Dental School, Hamadan University of Medical Sciences. These teeth had no cracks, carious lesions or coronal restorations. The teeth were cleaned with a periodontal curette and immersed in 10% formalin (Shahid Ghazi Co., Tabriz, Iran) for disinfection for 1 week at room temperature. The teeth were placed in distilled water (Shahid Ghazi Co.) 24 hours before use. The roots were separated from the crowns at CEJ using a #878-016 M diamond bur (PIRANHA DIA 2X 878-016, SS White, Lake Woods, NJ, USA) in a high-speed handpiece under air and water spray. The coronal pulps of the teeth were removed. The pulp chambers were filled with lightbody elastomeric impression material to avoid penetration of acrylic monomers into them. The samples were fixed in 25 × 35 × 10 mm molds containing translucent acrylic resin (Marlic Medical Industry Co., Tehran, Iran) with their labial surfaces facing up. The samples were kept in cold water until the acrylic resin was completely cured to control thermal affects generated by acrylic resin setting reaction. The labial surfaces of the samples were polished with wet 400- and 600-grit silicon carbide papers, respectively, to achieve flat homogeneous enamel surfaces without exposing of dentin in all the samples. The samples were stored in de-ionized water after polishing. Then, the samples were equally and randomly divided into 9 groups as follows:
G1: control (no bleaching); G2: bleaching and storage for 24 hours; G3: bleaching and storage for seven days; G4 - 6: bleaching and application of 600, 800 and 1,000 µmol EGCG-containing solution for 10 minutes, respectively; G7 - 9: bleaching and application of 600, 800 and 1,000 µmol EGCG-containing solution application for 20 minutes, respectively.
All the specimens except for the control group (no bleaching) specimens were subjected to a bleaching procedure. A bleaching gel (Opalescence Xtra Boost, Ultradent Products, Inc., South Jordan, UT, USA) containing 40% hydrogen peroxide was applied on the enamel surface for 10 minutes according to manufacturer's instructions. After the bleaching procedure was completed the samples were rinsed with water and dried with air spray.
For application of EGCG, the samples in groups 4 - 6 were immersed in 600, 800 and 1,000 µmol EGCG solutions for 10 and in groups 7 - 9 for 20 minutes, rinsed under running water for 1 minute and dried gently.
For composite resin build-up of all the groups, translucent cylindrical molds measuring 4 mm in diameter and 6 mm in length were fixed to sample surfaces. Then, 37% phosphoric acid gel (Ultra Etch, Ultradent Products, Inc.) was applied on every sample surface for 30 seconds, rinsed for 15 seconds and dried for 10 seconds. Then, a fifth-generation bonding agent (Adper Single Bond Plus Adhesive, 3M ESPE, St. Paul, MN, USA) was applied to the etched surface in two layers by a microbrush, dried for 5 seconds by a gentle air spray and irradiated for 20 seconds at a distance of 1 mm by a light-curing unit (Hilux LEDMAX 550, Benlioglu Dental, Ankara, Turkey). A light-cured composite resin (Filtek Z250, 3M ESPE) was placed into the molds using the incremental technique in 1-mm layers and each layer was light-cured for 20 seconds from two sides of the transparent mold. After removal of the mold, the samples were placed in distilled water for 24 hours at room temperature for completion of polymerization, except for samples in group 3, which were placed in distilled water for 1 week.
Shear bond strength test was performed by using a chisel-shaped crosshead in a universal test machine (Zwick 1446, Zwick, Ulm, Germany). The machine was set at a 50-kg scale reading at a crosshead speed of 0.5 mm/min. Fractured surfaces were observed using a stereomicroscope (Eclips E600, Nikon, Tokyo, Japan) under ×20 magnification. Failures were scored as cohesive (failure within the enamel or composite), adhesive (failure between the enamel and composite), or as mixed (mixture of the both).
Two specimens from each group were selected for Scanning Electron Microscope (SEM) observation to assess morphology of fractured surfaces. The specimens were ultrasonically cleaned for 5 minutes in deionized water (CP104, CEIA Int., Paris, France), immersed in 95% ethanol, and gently air-dried. Each specimen was gold sputter-coated (SC7620, Quorum Technologies Ltd., East Sussex, UK) and evaluated under a SEM (JSM-5310, JEOL, Tokyo, Japan) at different magnifications.
Data were analyzed with SPSS (version 15, SPSS Inc., Chicago, IL, USA ), using two-way ANOVA and Tukey HSD tests. Significance level was set at 0.5.
Table 1 presents means and standard deviations of shear bond strength values (MPa) in the 9 studied groups. Two-way ANOVA showed no significant effect of the main factors of time (F = 2.310, p > 0.05) and concentration (F = 1.088, p > 0.05) and their interactive effect (F = 0.088, p > 0.05) on bond strength of the groups. The results of one-way ANOVA indicated significant differences between positive (G1 and G3), negative (G2) and EGCG groups (p < 0.05).
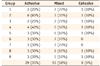
Post hoc Tukey HSD test showed no significant differences between groups (p > 0.05) except for group 2 and other groups. Stereomicroscopic assessment of the fractured surfaces is summarized in Table 2. In addition, SEM observation demonstrated porosities in the resin layer on the bonded enamel surface in group 2. In addition, there was a uniform resin layer on the bonded enamel surface in group 1 (control) and groups 5 and 6 (Figures 1, 2, 3, 4).
Based on the results of the present study, group 2 exhibited the lowest bond strength values compared to other groups. Some studies have shown physical changes in the enamel structure due to the effect of bleaching agents and decreases in bond strength have been attributed to an increase in enamel surface porosity as a result of overetching and loss of the crystalline structure of enamel.7 Recently, a study has shown that a decrease in bond strength subsequent to the application of bleaching agents in enamel surfaces is reversible in the form of a decrease in the calcium content of enamel, a decrease in surface hardness of enamel and disintegration of enamel structure.19 Rotstein et al. reported that strength and solubility of enamel, dentin and cementum decrease after bleaching. An increase in solubility is attributed to changes in the organic and mineral structure of teeth.20 Some other studies have reported an increase in enamel porosity to retain oxygen in the form of free radicals.21 Generally, the results of the present study are accordance with those of studies carried out by Kaya et al., Bulut et al. and Khoroushi et al.6,8,11
It has been suggested that storage of bleached samples in saliva or a moist environment can gradually release this oxygen reservoir in the form of a solution or a gas to eliminate it completely. Therefore, bond strength values in group 3, in which no antioxidants were used, were significantly different from those in group 2 but were not significantly different from those in the control group and groups 4 - 9. Several studies have concluded that a one-week delay in the bonding procedure is sufficient to compensate the negative effects of bleaching on bond strength.22,23 However, some studies have suggested a delay of 2 - 3 weeks. It appears differences in results can be attributed to differences in bleaching techniques, the bleaching agent and the substrate.7,25 The results of the present study showed that use of EGCG, in spite of concentration and duration of application, can result in a significant increase in bond strength, with no significant differences from the control group. It seems that these results are due to the antioxidative activity of EGCG, which is related to its polyphenolic nature and chemical composition. Each molecule of this material contains four rings of A, B, C and D. Studies have shown that trihydroxy and dihydroxy groups of B ring are responsible for the antioxidative properties of the material. Like every antioxidative agent, polyphenols prevent the formation of oxygen free radicals.24,26-28 Antioxidants neutralize reactive radicals by donating one of their electrons to them. However, neutral antioxidants are not converted into free radicals because they are stable in this form.29,30
It has been shown that polyphenols in green tea inhibit the oxidative activity in rats up to 72%.28 Reductive activity of EGCG has been shown in medical studies.5,14,15,27,28
A large number of studies on this material, as the most abundant catechin in green tea, have shown its benefits for human body and it appears it does not have any detrimental effects on orodental structures.31-33
Since no studies have been carried out on the antioxidative activity of this polyphenol in green tea in dentistry, it is expected that its use on tooth structures after bleaching might neutralize the effect of oxygen free radicals retained in tooth structures and improve the bond strength of composite resin to tooth structure. However, biochemical studies are necessary to confirm this theory.
The results of the present study showed no significant differences between groups 4 - 9, in which three different concentrations of the solution were used. Türkün indicated that sodium ascorbate gel at concentrations less than 10% cannot reverse the decreased bond strength of bleached teeth; they reported concentration of sodium ascorbate to be a more important factor compared to the form of the material used (solution vs. gel).34 However, Dabas et al. and Kimyai et al. showed that 10% and 20% sodium ascorbate were not significantly different in increasing bond strength.21,35 It appears there should be a minimum threshold concentration for the antioxidative effect of materials. Therefore, the results of the present study showed that 600 µmol concentration of EGCG can exhibit favorable effect to increase bond strength. In the present study, 600, 800 and 1,000 µmol concentrations of EGCG were used. The basic concentration used in previous studies was applied in the present study.17,18 In some studies 16%, 20% and 22% carbamide peroxide has been used and in other studies hydrogen peroxide with a higher concentration has been applied.36,37 In this study, 40% hydrogen peroxide was used as an external bleaching agent.
Since the present study was a preliminary study in this respect, the solution form of EGCG was used by immersion. A large number of studies have been carried out in order to determine the form of antioxidant that should be used, some of which have preferred the hydrogel form due to the better control and ease of application.38,39
The solutions containing different concentrations of EGCG were colorless and enamel discoloration was not seen during the procedures.
The duration of EGCG application did not result in significant differences in shear bond strength values, indicating that this material can provide sufficient antioxidative effect in at least 10 minutes. Since there were no studies to determine the minimum application time of EGCG to bleached enamel, the duration of 10 minutes was selected based on study results of Türkün et al. and Kaya et al.4,8
Selection of a 20-minute duration in the present study was based on a study by Torres, although in a study by Kaya et al. longer times of 60, 120, 240 and 480 minutes have been used.10,40 However, a 10-minute application of the antioxidant in this study resulted in a significant increase in bond strength; this duration is applicable in clinical situations.
In the present study, the solution form of the material was used by immersion. It is recommended that other forms of this material, e.g. hydrogel form, which are clinically more acceptable and controllable, be prepared and compared with the solution form.
In the present study, thermocycling was not carried out. Future studies with thermocycling might yield more accurate results. The present study was a preliminary study; therefore, designing a comparative study with other routine antioxidants used to improve bond strength is recommended.
Figures and Tables
Figure 1
SEM views of the fractured surface in group 2, showing porosities in the resin layer on the bonded enamel surface (P, porosity). (a) ×250; (b) ×5,000.

Figure 2
SEM views of the fractured surface in group 1 (control), showing a uniform resin layer on the bonded enamel surface. (a) ×250; (b) ×5,000.

Figure 3
SEM views of the fractured surface in group 6, showing a uniform resin layer on the bonded enamel surface. (a) ×250; (b) ×5,000.

Figure 4
SEM views of the fractured surface in group 3, showing a uniform resin layer on the bonded enamel surface. (a) ×250; (b) ×5,000.

Acknowledgements
This article was prepared from a dissertation for a specialty degree in restorative dentistry. The authors would like to extend their gratitude to the Deputy of Research at Hamadan University of Medical Sciences and the Dental Research Center for the financial support provided.
References
1. Garcia EJ, Oldoni TL, Alencar SM, Reis A, Loguercio AD, Grande RH. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J. 2012; 23:22–27.

3. Heymann HO. Additional Conservative Esthetic Procedure. Sturdevant's Art and Science of Operative Dentistry. 6th ed. Philadelphia: Elsevier Health Sciences;2013. Chapter 12.
4. Türkün M, Kaya AD. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J Oral Rehabil. 2004; 31:1184–1191.

5. Coyle CH, Philips BJ, Morrisroe SN, Chancellor MB, Yoshimura N. Antioxidant effects of green tea and its polyphenols on bladder cells. Life Sci. 2008; 83:12–18.

6. Khoroushi M, Feiz A, Khodamoradi R. Fracture resistance of endodontically-treated teeth: effect of combination bleaching and an antioxidant. Oper Dent. 2010; 35:530–537.

7. Uysal T, Ertas H, Sagsen B, Bulut H, Er O, Ustdal A. Can intra-coronally bleached teeth be bonded safely after antioxidant treatment? Dent Mater J. 2010; 29:47–52.

8. Kaya AD, Türkün M. Reversal of dentin bonding to bleached teeth. Oper Dent. 2003; 28:825–829.
9. Cavalli V, Reis AF, Giannini M, Ambrosano GM. The effect of elapsed time following bleaching on enamel bond strength of resin composite. Oper Dent. 2001; 26:597–602.
10. Kaya AD, Türkün M, Arici M. Reversal of compromised bonding in bleached enamel using antioxidant gel. Oper Dent. 2008; 33:441–447.

11. Bulut H, Turkun M, Kaya AD. Effect of an antioxidizing agent on the shear bond strength of brackets bonded to bleached human enamel. Am J Orthod Dentofacial Orthop. 2006; 129:266–272.

12. Muraguchi K, Shigenobu S, Suzuki S, Tanaka T. Improvement of bonding to bleached bovine tooth surfaces by ascorbic acid treatment. Dent Mater J. 2007; 26:875–881.

13. Sasaki RT, Flório FM, Basting RT. Effect of 10% sodium ascorbate and 10% alpha-tocopherol in different formulations on the shear bond strength of enamel and dentin submitted to a home-use bleaching treatment. Oper Dent. 2009; 34:746–752.

14. Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010; 501:65–72.

15. Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003; 133:3275S–3284S.
16. Huang CC, Wu WB, Fang JY, Chiang HS, Chen SK, Chen BH, Chen YT, Hung CF. Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules. 2007; 12:1845–1858.

17. Kato MT, Magalhães AC, Rios D, Hannas AR, Attin T, Buzalaf MA. Protective effect of green tea on dentin erosion and abrasion. J Appl Oral Sci. 2009; 17:560–564.

18. Naderi NJ, Niakan M, Kharazi Fard MJ, Zardi S. Antibacterial activity of Iranian green and black tea on streptococcus mutans: an in vitro study. J Dent (Tehran). 2011; 8:55–59.
19. Poorni S, Kumar RA, Shankar P, Indira R, Ramachandran S. Effect of 10% sodium ascorbate on the calcium: Phosphorus ratio of enamel bleached with 35% hydrogen peroxide: an in vitro quantitative energydispersive Xray analysis. Contemp Clin Dent. 2010; 1:223–226.
20. Rotstein I, Lehr Z, Gedalia I. Effect of bleaching agents on inorganic components of human dentin and cementum. J Endod. 1992; 18:290–293.

21. Dabas D, Patil AC, Uppin VM. Evaluation of the effect of concentration and duration of application of sodium ascorbate hydrogel on the bond strength of composite resin to bleached enamel. J Conserv Dent. 2011; 14:356–360.

22. Barcellos DC, Benetti P, Fernandes VV Jr, Valera MC. Effect of carbamide peroxide bleaching gel concentration on the bond strength of dental substrates and resin composite. Oper Dent. 2010; 35:463–469.

23. Feiz A, Khoroushi M, Gheisarifar M. Bond strength of composite resin to bleached dentin: effect of using antioxidant versus buffering agent. J Dent (Tehran). 2011; 8:60–66.
24. Roy P, George J, Srivastava S, Tyagi S, Shukla Y. Inhibitory effects of tea polyphenols by targeting cyclooxygenase-2 through regulation of nuclear factor kappa B, Akt and p53 in rat mammary tumors. Invest New Drugs. 2011; 29:225–231.

25. Kunt GE, Yılmaz N, Sen S, Dede DÖ. Effect of antioxidant treatment on the shear bond strength of composite resin to bleached enamel. Acta Odontol Scand. 2011; 69:287–291.

26. Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002; 42:25–54.

27. Ho CT, Chen Q, Shi H, Zhang KQ, Rosen RT. Antioxidative effect of polyphenol extract prepared from various Chinese teas. Prev Med. 1992; 21:520–525.

28. Kanwar J, Taskeen M, Mohammad I, Huo C, Chan TH, Dou QP. Recent advances on tea polyphenols. Front Biosci (Elite Ed). 2012; 4:111–131.

29. Carnelio S, Khan SA, Rodrigues G. Definite, probable or dubious: antioxidants trilogy in clinical dentistry. Br Dent J. 2008; 204:29–32.

31. Suzuki Y, Miyoshi N, Isemura M. Health-promoting effects of green tea. Proc Jpn Acad Ser B Phys Biol Sci. 2012; 88:88–101.

32. Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol Nutr Food Res. 2011; 55:832–843.

33. Suganuma M, Saha A, Fujiki H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011; 102:317–323.

34. Türkün M, Celik EU, Kaya AD, Arici M. Can the hydrogel form of sodium ascorbate be used to reverse compromised bond strength after bleaching? J Adhes Dent. 2009; 11:35–40.
35. Kimyai S, Valizadeh H. Comparison of the effect of hydrogel and a solution of sodium ascorbate on dentin composite bond strength after bleaching. J Contemp Dent Pract. 2008; 9:105–112.

36. Dominici JT, Eleazer PD, Clark SJ, Staat RH, Scheetz JP. Disinfection/sterilization of extracted teeth for dental student use. J Dent Educ. 2001; 65:1278–1280.

37. Lima AF, Fonseca FM, Freitas MS, Palialol AR, Aguiar FH, Marchi GM. Effect of bleaching treatment and reduced application time of an antioxidant on bond strength to bleached enamel and subjacent dentin. J Adhes Dent. 2011; 13:537–542.
38. Nour El-din AK, Miller BH, Griggs JA, Wakefield C. Immediate bonding to bleached enamel. Oper Dent. 2006; 31:106–114.

39. Kimyai S, Valizadeh H. The effect of hydrogel and solution of sodium ascorbate on bond strength in bleached enamel. Oper Dent. 2006; 31:496–499.

40. Torres G, Koga A, Borges A. The effects of anti-oxidants as neutralizers of bleaching agents on enamel bond strength. Braz J Oral Sic. 2006; 5:971–975.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download