Abstract
Objectives
The purpose of the study was to evaluate human dental pulp response to pulpotomy with calcium hydroxide (CH), mineral trioxide aggregate (MTA), and calcium enriched mixture (CEM) cement.
Materials and Methods
A total of nine erupted third molars were randomly assigned to each pulpotomy group. The same clinician performed full pulpotomies and coronal restorations. The patients were followed clinically for six months; the teeth were then extracted and prepared for histological assessments. The samples were blindly assessed by an independent observer for pulp vitality, pulp inflammation, and calcified bridge formation.
Results
All patients were free of clinical signs/symptoms of pulpal/periradicular diseases during the follow up period. In CH group, one tooth had necrotic radicular pulp; other two teeth in this group had vital uninflamed pulps with complete dentinal bridge formation. In CEM cement and MTA groups all teeth had vital uninflamed radicular pulps. A complete dentinal bridge was formed beneath CEM cement and MTA in all roots. Odontoblast-like cells were present beneath CEM cement and MTA in all samples.
A classic study by Kakehashi et al. demonstrated the crucial role of the bacteria in development of endodontic diseases. In a germ-free environment, the pulp demonstrated the ability to heal and produce reparative dentin. This is a basic principle for success of all vital pulp therapies.1-2
Pulpotomy is a vital pulp therapy in which the coronal pulp is removed partially or completely and the remaining pulp is covered with a pulpotomy agent.3 This treatment is based on the rational that after amputation of coronal pulp of the permanent teeth, the remaining pulp is protected by a hard-tissue stimulating material, and remains vital and healthy.4 Several materials have been proposed as pulpotomy agents such as calcium hydroxide (CH), adhesive systems, and mineral trioxide aggregate (MTA).5-8 Studies showed that a material used as a pulp protecting agent should be biocompatible, be able to induce hard tissue formation, bactericidal, and create a long-lasting biological seal.2,8
Historically, CH has been the material of choice for vital pulp therapies. CH is an alkaline material with well documented bactericidal properties and the ability to induce hard-tissue formation in human teeth.5 However, CH has several disadvantages including poor adherence to dentin and poor sealing ability, unpredictable dentinal bridge formation, and the presence of tunnel defects in these bridges which may act as potential pathways for bacterial leakage.8-11
Use of MTA as a pulpotomy agent in permanent teeth has demonstrated excellent histological and clinical results.4,7,12,13 MTA has demonstrated acceptable biocompatibility, sealing ability and the ability to promote healing in the pulp tissue.8,14-16 Hard-tissue induction by MTA has been documented in several animal and human studies.7,8,10-13 However, compared with CH, the antibacterial activity of MTA is inferior.17 In addition, the potential for discoloration, delayed setting time, and high cost are other disadvantages of MTA.18
Recently, a new endodontic biomaterial, calcium enriched mixture (CEM) cement, has been introduced as a water-based tooth-colored cement with similar clinical applications to MTA but different chemical composition.19,20 The major components of the CEM cement powder are CaO, SO3, P2O5 and SiO2, and the minor components are Al2O3, Na2O, MgO, Cl. This cement is alkaline (pH > 10.5) and releases CH during and after setting.19,20 Several ex vivo and in vivo studies have documented biocompatibility of CEM cement.10,21-24 Antibacterial activity of CEM cement against endodontic pathogens is comparable to CH and superior to MTA, and its sealing ability is similar to MTA.17,25 The ability of CEM cement to induce hard tissue formation has been documented histologically and radiographically.10,22,26-28 Successful results were obtained when CEM cement was used as a pulpotomy agent in human immature and mature permanent teeth.27-31
Whilst there has been a randomized controlled trial in a dog model which compared the pulpal reaction to CH as a traditional pulpotomy agent histologically, with MTA as the gold standard and CEM as a novel endodontic biomaterial, there has been no study to examine the effect of these materials on human dental pulp.22 The aim of this paper was to investigate, histologically, the effect of CH, MTA and CEM cement on human dental pulp as pulpotomy agents.
This project was evaluated and approved by Ethics Committee of Iranian Center for Endodontic Research in Shahid Beheshti University of Medical Sciences. Adult volunteers were considered for the study, each had at least three healthy and clinically erupted third molars scheduled for extraction because of orthodontic/prosthetic reasons. Inclusion criteria were as the following: clinically normal pulp; normal response to percussion and palpation; apices were closed; and the periodontal condition was normal (probing depths < 3 mm). Exclusion criteria were caries or history of restoration, pulp or canal calcification, apical radiolucency, and teeth which could not be isolated with a rubber dam. Volunteers were not medically compromised. After thoroughly explaining the clinical procedures and risks involved, informed consent was obtained from each volunteer, for each tooth.
A total of 9 clinically erupted third molars from 3 volunteers were included in the study. Volunteers were all healthy males at the age of 22 to 24. For each tooth, cold test using Endo-Frost cold spray (Roeko, Coltene Whaledent, Langenau, Germany), palpation and percussion were performed. In each volunteer, randomly, one tooth was assigned to one of the three experimental groups including CH, MTA, and CEM cement as pulpotomy agents.
The same pulpotomy technique was performed by the same clinician in all three experimental groups. Before initiating the procedure, all volunteers rinsed with 0.12% chlorhexidine mouth wash (Behsa Pharmaceutical Co., Tehran, Iran) for 30 seconds. After local anesthesia using 2% lidocaine and 1 : 80000 epinephrine (Darou Pakhsh Pharmaceutical Mfg Co., Tehran, Iran) and rubber dam isolation, the teeth were swabbed with 0.12% chlorhexidine (Behsa Pharmaceutical Co.). Access cavities were prepared using a diamond fissure bur (Diatech, Heerbrugg, Switzerland) and high speed handpiece with copious water spray. The pulps were amputated to the orifice level of the root canals using a long shank diamond round bur (Diatech) in a high speed handpiece with copious water spray to prevent heat damage to the subjacent pulp. Vitality of all included teeth was confirmed by presence of bleeding upon pulp exposure. Hemostasis was achieved by application of 5.25% NaOCl for up to 10 minutes in the access cavities. The radicular clot-free pulps were capped with one of the pulpotomy agents as follow. In CEM cement group (CEM cement, Bioniquedent, Tehran, Iran), the powder and liquid were mixed according to the manufacturer's instructions. The mixture was placed in the access cavity with a sterile amalgam carrier and adapted gently to the dentin walls by a moistened sterile cotton pellet. In MTA group (Tooth-colored ProRoot MTA, Dentsply Tulsa Dental Specialties Tulsa, OK, USA), the powder and liquid were mixed according to the manufacturer's instructions and pulps were capped as described above. In the CH group (Calcium Hydroxide, Aria Dent, Teheran, Iran), the powder and sterile normal saline were mixed to achieve putty consistency and pulps were capped using the same technique as other experimental groups.
Immediately after placement of the capping agent, a 2 mm thick layer of glass ionomer (GC lining, GC Corp., Tokyo, Japan) was placed over the pulp capping material, and the access cavity restored with amalgam (gs-80, SDI Ltd., Bayswater, Australia). A post-operative radiograph was taken to confirm the quality of procedure (Figure 1).
All experimental teeth were followed up clinically. Clinical assessments included a history of symptoms (if any), cold test using Endo-Frost cold spray (Roeko), palpation and percussion. Six months after operation all teeth were extracted atraumatically by an oral and maxillofacial surgeon.
Immediately after extraction the teeth were rinsed in cold water and roots were cut off at midroot level using a diamond fissure bur in high speed handpiece with water spray coolant to allow the fixative penetration. The specimens were coded for blind histological evaluations. The teeth were then placed in separate bottles containing 10% buffered formaldehyde for 120 days, and decalcified for 4 weeks in 10% formic acid. After decalcification, the specimens were rinsed with running tap water for 2 hours, followed by dehydration in ascending concentrations of alcohol (70%, 90%, and 100%), and were embedded in paraffin. The amalgam restorations and glass-ionomer bases were removed gently before embedding. Five µm labiolingual sections were prepared serially, and then specimens were stained with hematoxylin and eosin (H&E). Using an optical microscope (Lichtmikroskope, Carl Zeiss, Göttingen, Germany), samples was evaluated by an experienced oral pathologist who was blinded to the type of pulpotomy agent. The amount of pulp inflammation (type, intensity, and extension), the quality of hard tissue formation beneath pulpotomy agent (continuity, morphology, and thickness), and other histological features including presence/absence of giant cells, calcifications, and odontoblast-like cells beneath pulpotomy material and the pattern of odontoblast-like cells (if present) were evaluated in all sections according to the standards previously described.32
Before the procedure, all included teeth were responsive to cold test and they were neither sensitive to palpation nor to percussion. None of the volunteers reported a history of pain or discomfort in the experimental teeth for the 6 months following the operative intervention. On clinical examination before extraction, no teeth were sensitive to palpation or percussion. All of the experimental teeth responded negatively to the cold test.
As after full pulpotomy pulps of the roots respond independently to the treatment, the histological assessments were done for each root separately. One tooth in the CEM cement group and two teeth in the MTA group had only one root canal for examination; the others were two rooted. The outcomes were as the following:
CH group: Vital pulp was seen in two teeth (Figure 2). One two rooted tooth (the same tooth shown in Figure 1), had necrotic pulp in all roots/sections; resorptive lacunas were seen in roots with necrotic pulp (Figure 3). A continuous calcified bridge was seen in all roots with vital pulp. There was no inflammation in roots with vital pulps (Figure 2). No evidence of odontoblast-like cells was found beneath CH in two roots with vital pulps. Diffuse calcification could be seen in one tooth (two roots).
CEM cement group: Vital pulp was seen in all roots (Figure 2). The calcified bridge formed beneath CEM cement was continuous in all specimens. Pulp tissues were normal without inflammation/hyperemia. There was no evidence of diffuse/focal calcification (Figure 2).
MTA group: Vital pulp was seen in all roots (Figure 2). The calcified bridge beneath MTA was continuous in all sections. Pulp tissues were normal. There was neither inflammation/hyperemia nor diffuse/focal calcification (Figure 2).
Table 1 demonstrates a summary of findings for all groups.
Recent clinical trials and case studies on pulpotomy with CEM cement demonstrated favorable outcomes. A case series study on CEM cement pulpotomy of mature carious exposed teeth revealed 100% clinical and radiographic success rate after a mean follow up period of 15.8 months.27 A randomized clinical trial on pulpotomy of immature carious exposed first molars with MTA and CEM cement showed 100% clinical and radiographic rates for both biomaterials after 12 months.29 The similar results were obtained in pulpotomy of mature carious exposed human teeth with MTA (95% overall success rate) and CEM cement (92% overall success rate) after 12 months follow up.31 In addition, while comparison of the clinical outcomes of conventional root canal therapy and pulpotomy with CEM cement in human carious exposed mature teeth revealed similar outcomes for both treatments, the radiographic outcomes of the pulpotomy with CEM cement were significantly better than root canal therapy after one year.30
Although there are several clinical studies on outcomes of pulpotomy treatment in human teeth, the histological data are limited. A comparative study on human dental pulp response to CH and MTA revealed that pulps capped with MTA produced more homogeneous and more continuous dentinal bridges than pulps capped with CH.11 Eghbal et al. performed a histological study on twelve carious exposed human teeth with irreversible pulpitis which were scheduled for extraction.12 The histological assessments were conducted two months after MTA pulpotomy of the teeth. The outcomes revealed that all pulps were vital without inflammation and a complete dentinal bridge was formed in all specimens. Another histological study by Chueh and Chiang on a case of carious exposed second premolar which had undergone MTA pulpotomy 10 months before extraction revealed a normal pulp and presence of a dentinal bridge beneath MTA.13
While formation of a calcified bridge beneath MTA has been shown in several clinical and histological studies on human teeth after vital pulp therapies, the data were limited about CEM cement in this regard. Animal studies on pulp capping and pulpotomy with CEM cement revealed that the dentinal bridges formed beneath CEM cement were similar to that formed beneath MTA regarding the thickness, continuity, and presence of odontoblast-like cells.10,22 Formation of a calcified bridge beneath CEM cement in human teeth was documented radiographically in case studies.26,28 A recent histological study on the responses of human dental pulp to direct pulp capping with CEM cement demonstrated that there was no significant difference between CEM cement and MTA regarding the morphology of dentinal bridges formed beneath pulp capping material and pulpal inflammation.33 The mean thickness of dentinal bridges in CEM cement group was greater than MTA; however the difference was not significant.33 The present study is the first histological study aimed to evaluate human dental pulp reaction to pulpotomy with CEM cement. The pulpal reactions to CEM cement were indicative of its biocompatibility.
Formation of a hard tissue barrier beneath a pulpotomy agent acts as a protective pulp barrier and is an indicator of pulp health and vitality.34 MTA is a bioactive material which releases calcium ions during and after setting. The calcium ions react with environmental phosphorus and produce hydroxyapatite crystals on the surface of MTA and MTA-dentin interface.35,36 The bioactivity of MTA is considered to be responsible for its biocompatibility, dentinogenic activity and sealing ability.35 On the other hand, CEM cement is a bioactive material with indigenous reservoir of phosphorus ions. It means that CEM cement is able to produce hydroxylapatite crystals in phosphorus free environments like normal saline.36 Therefore, like MTA, bioactivity of CEM cement might be responsible for its biocompatibility, hard tissue induction potential, and sealing ability. In addition, the surface characteristics of set CEM cement is similar to human dentin, for example the distribution pattern of calcium, phosphorus, and oxygen ions.19 Consequently, there might be a possibility that bioactivity and surface features of the set cement induce pulpal cells to produce dentinal bridge beneath CEM cement.
Histological evaluations of pulpal responses to vital pulp therapy with CH demonstrated that the dentinal bridge formation beneath CH was unpredictable. Moreover, unlike pulps capped with MTA, pulps that were capped with CH were inflamed.8 Animal studies revealed that, in comparison to MTA and CEM cement, dentinal bridges formed beneath CH were in lower quality in terms of thickness and presence of tunnel defects.10,22 Pulpal inflammation, dystrophic calcifications, and foci of necrosis were other unfavorable findings in CH groups. Findings of the present study showed that the human dental pulp response to CH is not predictable. While, two teeth out of three demonstrated favorable responses, one tooth showed pulp necrosis without dentinal bridge formation. However, due to small sample size, statistical analysis was not done.
Another interesting finding was that the necrotic tooth in CH group didn't show any clinical signs/symptoms of pulpal or periradicular diseases during 6 months after pulpotomy. Studies have shown that success rate of vital pulp therapy with CH decreases overtime.37 These findings show the importance of long-term follow ups after vital pulp therapies, specifically when the material used is CH.
MTA and CEM cement are water-based cements which need aqueous environments to set.20,38,39 Therefore, the clinician should prepare enough moisture for the cement to set before continuation of the procedure which increases the treatment time. Comparing to vital pulp therapy with CH, this issue is a disadvantage for water-based cements. In the present study, the clinician didn't provide the water-based cements with further moisture and the pulpotomy materials were covered with a layer of glass ionomer immediately after insertion. The rational was that it was possible for the water-based cements to obtain necessary moisture for setting reactions from wounded pulp tissue. The favorable histological outcomes of the study show that this might be a practical method for utilizing water-based cements in pulpotomy treatments and helps to reduce the treatment time. However, further studies are recommended.
Although the outcomes of the present study shows favorable biological properties of CEM cement, it should be noted that the pulpotomies were done on healthy human teeth without history of caries or restoration. Presence of inflammation in human pulp tissue due to the history of caries/restoration, or carious pulpal exposure reduces the population of dental pulp stem cells (DPSCs) as well as their osteogenic/dentinogenic potential.40 These findings raise questions about the healing potential of inflamed pulp tissue. However, in the study by Alongi et al. it was shown that tissue regeneration potential (i.e. formation of pulp-dentin complex) of DPSCs from inflamed pulps with irreversible pulpitis was similar to DPSCs from normal pulps.40 It should be noted that these findings are not yet confirmed clinically. This subject needs further studies.
Figures and Tables
Figure 1
Preoperative and postoperative periapical radiographs of clinically erupted third molars; postoperative radiographs were taken immediately after operation to confirm the quality of pulpotomies and coronal restorations. CH, calcium hydroxide; MTA, mineral trioxide aggregate; CEM, calcium enriched mixture.
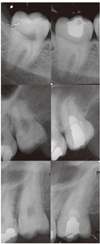
Figure 2
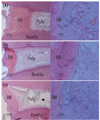
Composite photomicrograph of radicular pulps of healthy maxillary/mandibular erupted third molars six months after full pulpotomy with (a) calcium hydroxide (CH); (b) calcium enriched mixture (CEM) cement; (c) mineral trioxide aggregate (MTA) (Hematoxylin and Eosin staining, The magnifications of the micrographs in the left and right columns are ×100 and ×400, respectively). Clinically, all teeth were functional without sensitivity to percussion/palpation during the time period after pulpotomy. Note the continuous dentinal bridges (DB) beneath pulpotomy agents with regular tubular pattern and presence of odontoblast-like cells adjacent to dentinal bridges.
Acknowledgement
This study was supported by the School of Dental Medicine, University of Connecticut Health Center, Farmington, Connecticut; and the Iranian Center for Endodontic Research, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The authors gratefully acknowledge the contribution of Professor Kamran E. Safavi for his histological preparations and his helpful comments on the manuscript. The authors acknowledge the contributions of Drs. S. Soltani, M. Ghafoori, and M. Hatami in this study.
References
1. Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965; 20:340–349.

2. Witherspoon DE. Vital pulp therapy with new materials: new directions and treatment perspectives-permanent teeth. J Endod. 2008; 34:S25–S28.

3. Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000; 28:77–92.

4. Witherspoon DE, Small JC, Harris GZ. Mineral trioxide aggregate pulpotomies: a case series outcomes assesment. J Am Dent Assoc. 2006; 137:610–618.
5. Nosrat IV, Nosrat CA. Reparative hard tissue formation following calcium hydroxide application after partial pulpotomy in cariously exposed pulps of permanent teeth. Int Endod J. 1998; 31:221–226.

6. Costa CA, Hebling J, Hanks CT. Current status of pulp capping with dentin adhesive systems: a review. Dent Mater. 2000; 16:188–197.

7. Barrieshi-Nusair KM, Qudeimat MA. A prospective clinical study of mineral trioxide aggregate for partial pulpotomy in cariously exposed permanent teeth. J Endod. 2006; 32:731–735.

8. Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996; 127:1491–1494.

9. Holland R, de Souza V, de Mello W, Nery MJ, Bernabé PF, Otoboni Filho JA. Permeability of the hard tissue bridge formed after pulpotomy with calcium hydroxide: a histologic study. J Am Dent Assoc. 1979; 99:472–475.

10. Asgary S, Eghbal MJ, Parirokh M, Ghanavati F, Rahimi H. A comparative study of histologic response to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:609–614.

11. Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008; 41:128–150.

12. Eghbal MJ, Asgary S, Baghlue RA, Parirokh M, Ghoddusi J. MTA pulpotomy of human permanent molars with irreversible pulpitis. Aust Endod J. 2009; 35:4–8.

13. Chueh LH, Chiang CP. Histology of Irreversible pulpitis premolars treated with mineral trioxide aggregate pulpotomy. Oper Dent. 2010; 35:370–374.

14. Yang WK, Ko HJ, Kim MR. Evaluation of the rat tissue reaction to experimental new resin cement and mineral trioxide aggregate cement. Restor Dent Endod. 2012; 37:194–200.

15. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review-part II: leakage and biocompatibility investigations. J Endod. 2010; 36:190–202.

16. Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J Endod. 2011; 37:562–567.

17. Asgary S, Kamrani FA. Antibacterial effects of five different root canal sealing materials. J Oral Sci. 2008; 50:469–474.

18. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413.

19. Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J, Kheirieh S, Brink F. Comparison of mineral trioxide aggregate's composition with Portland cements and a new endodontic cement. J Endod. 2009; 35:243–250.

20. Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The Properties of a New Endodontic Material. J Endod. 2008; 34:990–993.

21. Mozayeni MA, Milani AS, Marvasti LA, Asgary S. Cytotoxicity of calcium enriched mixture cement compared with mineral trioxide aggregate and intermediate restorative material. Aust Endod J. 2012; 38:70–75.

22. Tabarsi B, Parirokh M, Eghbal MJ, Haghdoost AA, Torabzadeh H, Asgary S. A comparative study of dental pulp response to several pulpotomy agents. Int Endod J. 2010; 43:565–571.

23. Asgary S, Nosrat A, Homayounfar N. Periapical healing after direct pulp capping with calcium-enriched mixture cement: a case report. Oper Dent. 2012; 37:571–575.

24. Parirokh M, Mirsoltani B, Raoof M, Tabrizchi H, Haghdoost AA. Comparative study of subcutaneous tissue responses to a novel root-end filling material and white and grey mineral trioxide aggregate. Int Endod J. 2011; 44:283–289.

25. Asgary S, Eghbal MJ, Parirokh M. Sealing ability of a novel endodontic cement as a root-end filling material. J Biomed Mater Res A. 2008; 87:706–709.

26. Nosrat A, Asgary S. Apexogenesis treatment with a new endodontic cement: a case report. J Endod. 2010; 36:912–914.
27. Asgary S, Ehsani S. Permanent molar pulpotomy with a new endodontic cement: a case series. J Conserv Dent. 2009; 12:31–36.

28. Nosrat A, Asgary S. Apexogenesis of a symptomatic molar with calcium enriched mixture. Int Endod J. 2010; 43:940–944.

29. Nosrat A, Seifi A, Asgary S. Pulpotomy in caries-exposed immature permanent molars using calcium-enriched mixture cement or mineral trioxide aggregate: a randomized clinical trial. Int J Paediatr Dent. 2013; 23:56–63.

30. Asgary S, Eghbal MJ, Ghoddusi J, Yazdani S. One-year results of vital pulp therapy in permanent molars with irreversible pulpitis: an ongoing multicenter, randomized, non-inferiority clinical trial. Clin Oral Investig. 2013; 17:431–439.

31. Asgary S, Eghbal MJ. Treatment outcomes of pulpotomy in permanent molars with irreversible pulpitis using biomaterials: a multi-center randomized controlled trial. Acta Odontol Scand. 2013; 71:130–136.

32. Dominguez MS, Witherspoon DE, Gutmann JL, Opperman LA. Histological and scanning electron microscopy assessment of various vital pulp-therapy materials. J Endod. 2003; 29:324–333.

33. Zarrabi MH, Javidi M, Jafarian AH, Joushan B. Histologic assessment of human pulp response to capping with mineral trioxide aggregate and a novel endodontic cement. J Endod. 2010; 36:1778–1781.

34. Caliskan M. Clinical reliability of the dentine bridge formed after pulpotomy: a case report. Int Endod J. 1994; 27:52–55.

35. Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005; 31:97–100.

36. Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2009; 35:147–152.

37. Barthel CR, Rozenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 2000; 26:525–528.

38. Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995; 21:349–353.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download