Abstract
Objectives
The objective of this in vitro study was to evaluate and compare the cytotoxicity of four different root canal sealers i.e. Apexit Plus (Ivoclar Vivadent), Endomethasone N (Septodont), AH-26 (Dentsply) and Pulpdent Root Canal Sealer (Pulpdent), on a mouse fibroblast cell line (L929).
Materials and Methods
Thirty two discs for each sealer (5 mm in diameter and 2 mm in height) were fabricated in Teflon mould. The sealer extraction was made in cell culture medium (Dulbecco's Modified Eagle's Medium, DMEM) using the ratio 1.25 cm2/mL between the surface of the sealer samples and the volume of medium in a shaker incubator. Extraction of each sealer was obtained at 24 hr, 7th day, 14th day, and one month of interval. These extracts were incubated with L929 cell line and 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay was done. Two-way ANOVA for interaction effects between sealer and time and Post-hoc multiple comparison using Tukey's test across all the 16 different groups were used for statistical analysis.
Results
Apexit Plus root canal sealer was significantly less toxic than other sealers (p < 0.05) and showed higher cellular growth than control. Endomethasone N showed mild cytotoxicity. AH-26 showed severe toxicity which became mild after one month while Pulpdent Root Canal Sealer showed severe to moderate toxicity.
Root canal treatment aims to eliminate infection from the root canal and completely fill the root canal space in order to prevent apical and coronal penetration of liquids and microorganisms. Endodontic sealers are used to fill the gaps between the gutta-percha points and the root canal walls. It is widely recognized that sealers if extruded, may come in direct contact with periapical tissues and may affect them. Such conditions could cause degeneration of the tissue underlying the sealer and could also delay wound healing. Thus by current concept, sealers should be non-cytotoxic, non-mutagenic and immunologically compatible with periapical tissue.1,2
There are varieties of sealers with different physical and biological properties such as Pulpdent Root Canal Sealer, AH-26, Apexit Plus and Endomethasone N. Though manufacturers claim inertness of all these sealers, certain extracts or elutes has been found to extrude from them. Also degeneration products may gain access to periapical tissue through numerous pathways.3,4
This study was conducted to assess and compare the cytotoxicity of four sealers over a period of one month on mouse fibroblast cell line L929 by the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Method of the study was approved by regional ethical committee and study was conducted. An established cell line, mouse fibroblast L929 (American Type Culture Collection, ATCC, Manassas, VA, USA), was cultivated in 25 cm2 tissue flasks (Corning Life Sciences, St. Louis, MO, USA) containing Dulbecco's Modified Eagle's Medium (Hi-Media Laboratories Ltd., Mumbai, India). It was supplemented with 5% fetal calf serum (Sigma Aldrich Co., St. Louis, MO, USA), 100 IU/mL penicillin, 100 µg/mL streptomycin and 2 mmol/L L-glutamine (Hi-Media Laboratories Ltd.), and incubated in a humidified atmosphere of 95% air and 5% CO2 (Thermo Forma Incubator, Thermo Fisher Scientific, Mumbai, India) at 37℃. Subcultivation was performed with cells from sub-confluent cultures treated with 0.25% (w/v) Trypsin (Sigma Aldrich Co.), 0.02% ethylene diamine tetraaceticacid (EDTA, Hi-Media Laboratories Ltd.) and 0.05% glucose in phosphate buffer saline (TPVG, Hi-Media Laboratories Ltd.). After trypsinization, cells were seeded at a density of 5 × 104 cells per well in 96 well tissue culture plates and incubated for 24 hours in 5% CO2, 95% air at 37℃, to get sub confluent monolayers of cells.
This study evaluated four endodontic sealers such as calcium hydroxide based Apexit Plus (Group 1, Ivoclar Vivadent, Schaan, Liechtenstein), paraformaldehyde and corticosteroids based Endomethasone N (Group2, Septodont, Cedex, France), bisphenol epoxy resin based AH-26 (Group 3, Dentsply/De Trey, Konstanz, Germany), and zinc oxide eugenol based Pulpdent Root Canal Sealer (Group 4, Pulpdent Corporation, Watertown, MA, USA). The thirty two disc samples of each endodontic sealer were fabricated using Teflon mould having the dimension of 5 mm in diameter and 2 mm in height. All the samples of endodontic sealers were mixed aseptically according to manufacturer's instructions. The discs were allowed to set in humid chamber at 37℃ for 24 hours before the extraction procedure. The extract of sealers was made in cell culture medium (Dulbecco's Modified Eagle's Medium, DMEM) using the ratio 1.25 cm2/mL between the surface of the sealer samples and the volume of medium in a shaker incubator (Remi, Mumbai, India).
After 24 hours, the medium was removed and was designated as the first test solution. Another fresh DMEM was added with the same sealer disc in above ratio which was kept for another 7 days for extraction. Between 1st and 7th day the medium was renewed every second day and discarded. The last medium in contact with sealer for 24 hours was the test solution and was used to measure the cytotoxicity. At the end of 7th day the medium was removed which was labeled as the second test solution. With same method test solutions of 14th day and one month were collected. The pH of extracted solutions was measured by electronic pH meter (LI 127 PH meter, Elico Ltd, Hydrabad, India). The test solutions were filtered using Millex-GS sterile filter (Milipore S.A.S., Molsheim, Cedex, France). DMEM culture medium was used as control.
L929 Cells were diluted in fresh medium and seeded into 96 well plates (5 × 104 cells per well) (Corning Life Sciences). After incubation for 24 hours, the medium was aspirated from all wells and replaced with 100 µL per well of test solution or control medium. The test solutions were added to 32 wells per material per time period and two columns of wells plates (i.e. 16 wells) was filled with only culture medium as control group. The plates were incubated in an incubator (Thermo Forma, Thermo Fisher Scientific) before cytotoxicity was evaluated.
MTT (Sigma Aldrich Co.) solution was prepared as 1 mg/mL in phosphate buffer saline just before use. 100 µL MTT dye was added to each well containing cells treated with various extracts of sealers. Plates were incubated in a CO2 incubator for 3 hours. Optical density was determined by eluting the dye with dimethyl sulfoxide (Fisher Scientific, Pittsburgh, PA, USA), and the spectrophotometric absorbance was measured at 550 nm using a BioRad ELISA plate reader (Alfred Nobel Drive, Hercules, CA, USA).
Cytotoxicity was rated based on cell viability relative to control group, non cytotoxic > 90%, slightly cytotoxic 60 - 90%, moderately cytotoxic 30 - 59% and severly cytotoxic < 30% cell viability.5
The mean and standard deviation of these percentage values for each material and time periods were calculated. These values were analyzed statistically using parametric two-way ANOVA for interaction effect between sealer and time and post hoc Tukey's test were performed to determine the statistically significant differences across all the sixteen groups.
Table 1 shows the means and standard deviations of percent cell viability for different sealers with time. Two-way ANOVA demonstrated statistically significant interaction between sealer and time (p < 0.001, Figure 1), therefore post-hoc multiple comparison using Tukey's test was performed across all the 16 different groups with the results shown in Table 1. It is evident that irrespective of the sealer, the difference of mean cell viability after 24 hr and 7th day did not differ significantly; however, difference were observed from 14th day onwards for some sealers. For AH26 and Pulpdent root canal sealer, mean percent cell viability were significantly higher after 14th day. For Apexit Plus, significant difference was observed at 30th day as compared to previous time points. For Endomethasone N, the change was insignificant after 7th day. Further, irrespective of time, the mean percent cell viability for Apexit Plus was significantly higher than the other sealers.
In all period of time Apexit Plus showed no toxic effect on cells, while other sealers showed severe to mild toxicity. At one month, the order of cytotoxicity from severely toxic to non-toxic was Pulpdent Root Canal Sealer, AH-26, Endomethasone N, and Apexit Plus.
Clinically, root canal sealers are inserted into the root canal in a freshly mixed and incompletely polymerized stage, but even after the setting period, it is still possible that potentially toxic constituents may be released from the materials and leached into tissue fluids. For this reason, in the current study, cytotoxicity experiments were performed to estimate the cytotoxic potential of diffusible components of the set sealers.
L929 mouse fibroblasts is one of the most widely used cell line for in vitro assays. It is an ATCC certified and established cell line, which is readily available and gives reproducible results and hence, was used in this study for cytotoxicity evaluation.6,7
In this study, the extract of sealer was prepared as per ISO-10993-12 guidelines.8 Extracts from each sealer were obtained after 24 hours of mixing, when the sealer sets completely. The pH value of all extracts varied between 7.2 and 7.8, therefore the pH dependent alterations of cell metabolic activity was not of significance.9 MTT assay is a well established colorimetric assay for quantitative measurement of metabolically active cells.10 It focuses on the capacity of mitochondrial dehydrogenase enzymes in living cells to convert the yellow water soluble tetrazolium salt (MTT) into dark blue formazan crystals. Since dead cells are unable to produce the colored formazan product, this assay can be used to distinguish viable cells from dead cells. The amount of formazan formed is directly proportional to the mitochondrial enzyme activity in a given cell line. The advantages of this method is its simplicity, rapidity, and precision, in addition, it does not require radioisotopes.11,12
In present study all the four sealers were compared for the duration of one month. Various in vitro studies were performed previously on cytotoxicity of sealers for short period of time i.e. up to three weeks. In previous studies comparisons were done between only resin based sealers, between resin and ZOE based root canal sealers, or between above sealers with calcium hydroxide based sealers.6,9,13-20
Gerosa et al. and Schwartze et al. compared zinc oxide eugenol, calcium hydroxide, resin, and endomethasone based sealers.21,22 One of these studies sealer samples were kept with solution continuously for one week and other used freshly mixed sealers for 24 hours. When sealer is placed into the root canal the products leach in periapical area are constantly washed out by circulating blood, to simulate this condition during the preparation of extract for 7th day to one month, solution was renewed every second day and the solution which remains in contact for 24 hours only was used for cytotoxicity test. This procedure allows avoiding the increase concentration of leaching product into the solution when kept for long period of time.
In the present study, Apexit Plus root canal sealer showed no cytotoxic effect in all experimental time periods. In fact, it showed cellular viability even greater than that of control group. Guigand et al. observed that the calcium based material produced cell proliferation that reached 115% level as compared to control after 168 hours of exposure to the sealer.20 This stimulation of proliferation probably resulted from the liberation of calcium ions into the medium. It has been proved that free Ca++ ions have a favourable effect on cell proliferation.23-25 Also, Schwarze et al. observed no cytotoxic effect was caused by Apexit even when tested immediately after mixing.22 Their findings pointed out that no cell irritating components were released from setting Apexit. Geursten et al. showed similar favourable biocompatibility of calcium hydroxide sealers with more than 90% cell viability in culture.18
However, Leonardo et al. who evaluated microscopically for morphological changes in rat peritoneal macrophages for 72 hours and found that cell rupture and fragmentations were marked in culture tested with Apexit.9 This high toxicity was reported to be due to high alkalinity of the materials. Kim et al. compared freshly mixed sealers and found calcium hydroxide based sealer was more cytotoxic may be related to the method of applying the material to the cells.26
Endomethasone N showed mild cytotoxicity at all periods of time. These findings were supported by the studies performed by Schwarze et al. and Gerosa et al. Endomethasone N strongly inhibited the mitochondrial activity during the first 24 hours and significantly inhibited fibroblast viability up to 67% compared to control group.21,22,27 This finding was likely due to the release of eugenol from Endomethasone N.22 Schwarze et al. found 80% cell viability during initial periods of experiments and it increased to 100% within 26th week.27 Endomethasone N might disintegrate slowly in wet storage with liberation of cytotoxic components, such as eugenol or thymol.27
Gerosa et al. evaluated toxicity of 1st and 2nd week extract of Endomethasone and showed 85% cell viability of L929 fibroblasts in 1st week and nontoxicity in 2nd week.21 He attributed these findings to the presence of hydrocortisone in the sealer, which has known cytotoxic effects. Hence, it may be stated that Endomethasone contains eugenol, thymol and hydrocortisone, which was known as cytotoxic component.
AH-26 showed severe cytotoxicity at 24 hours and 7th day extract which became mild within one month. The initial toxicity of AH-26 may be due to the presence of formaldehyde which is released as a chemical byproduct of freshly mixed AH-26.16,17,28,29 Also Cohen et al. have suggested that AH-26 contains an epoxy resin component and that may be another cause of cytotoxicity for the material.30 They have also speculated that the amines in the composition of materials which accelerates polymerization may be related to toxicity of AH-26.
The most severe cytotoxic effects were observed in the 24 hours and 7th day extracts. These results of the present study were in agreement with Gerosa et al., Vajrabhaya et al., Koulaouzidou et al., and Miletic et al., who have found a similar pattern of cytotoxicity of AH-26.6,13,21,31 The pattern of cytotoxicity of AH-26 was in clear agreement with that reported by Spangberg et al. who showed formaldehyde (basic toxic component of AH-26) increased to nearly 200 times over the concentration of freshly mixed sealer within 24 hours, after that a relative reduction of formaldehyde was evident at the end of the 1st week.32 After the first week the amount of released formaldehyde was not significant. Similar result was also reported by Osorio et al.15
Pulpdent Root Canal Sealer is zinc oxide eugenol based and showed severe toxicity at the 24 hours and 7th day extract, which became moderate at the end of one month. Zinc oxide eugenol sealers are generally described in the literature as being relatively toxic to periapical tissues. It seems that continued solubility of zinc oxide eugenol and releasing of some unreacted ingredients, such as eugenol and Zn2+ are responsible for its severe long term cytotoxicity.16,19,20,26,28 Schwarze et al., found most pronounced cytotoxic effect of zinc oxide eugenol sealer on 3T3 cell metabolism during the first four weeks and Azar et al. observed similar results untill five weeks on human gingival fibroblasts.16,27 Zinc oxide eugenol sealers are highly water soluble, releasing high amount of potentially cytotoxic substances.33 By eliminating the eugenol from zinc oxide eugenol sealers, its toxicity will be reduced.34
The multiple comparisons for the percentage of cell viability were performed on all 16 different groups because there was a significant interaction effect between sealer and time. Pulpdent Root Canal Sealer and AH-26 showed no statistical differences at 24 hours and 7th day extract with marked decrease in cell viability. Pinna et al. and Key et al. found Zinc oxide eugenol was more cytotoxic than epoxy resins and calcium hydroxide based sealers.14,35 Further, Gerosa et al. and Miletic et al. noted that epoxy resin sealers were more toxic than endomethasone and calcium hydroxide sealers respectively.17,21
Despite the obvious cytotoxic effects of present study sealers, these products are commonly used in clinical practice. The animal experimental studies that have evaluated the effect of vital tissue exposure, reported limited tissue destruction, followed by tissue repair activity.36 Inflammatory activity together with intact blood supply in tissue repair process could reduce initial toxicity of material. It is important to note that a material that is hazardous in vitro may not necessarily be toxic in vivo.37 However, Cell culture systems may be of value in testing the biocompatibility of drugs, biomaterials or treatment techniques, as they allow the direct measurement of cytotoxicity and effect on cellular growth or the determination of tissue/material interactions. Thus in vitro studies help us to screen out toxic materials and let us know about its cytotoxic or genotoxic constituents. This information can be used for the further refinement and improvement of the material. Other methods used for determination of cell viability in culture are quantifying dsDNA (PicoGreen), ATP monitoring system (ATPLite), determination of protein content (BC Assay), determination of IL-6, IL-8 (ELISA) may provide greater accuracy of measuring cytotoxicity.38
This in vitro study has provided valuable insight into the cytotoxic activity of the commonly used sealers. Within the limitation of this study, it was concluded that, calcium hydroxide based sealers (Apexit Plus) have shown definite biocompatibility as compared to other formulations. Endomethasone N was showed mild toxicity in all periods of time. Cytotoxicty was maximum for the 24 hours and decreases over the period of time for resin based (AH-26) and zinc oxide eugenol based (Pulpdent Root Canal Sealer) sealer. Since cytotoxic effects of the other sealers were caused by their toxic ingredients, improvement of the chemical compositions of the present sealers could play a major role in reducing their toxicity.
Acknowledgements
We acknowledged Mr. Dhananjay Raje, Head Data Analysis Group MDS Bio-Analytics Pvt. Ltd. Nagpur, India for their support in statistical analysis.
References
1. Ingle JI, Backland KL. Chapter 30, Obturation of the radicular space. Endodontics. 6th ed. Hamilton: BC Decker Inc.;2008. p. 1053–1087.
2. Grossman LI, Oliet S, Del Rio CE. Chapter 9, Obturation of radicular space. Endodontic practice. 12th ed. New Delhi: Walter Kluwer Pvt. Ltd.;2010. p. 278–309.
3. De Deus QD. Frequency, location, and direction of lateral, secondary, and accessory canals. J Endod. 1975; 1:361–366. PMID: 10697487.
4. Dongari A, Lambrianidis T. Periodontally derived pulpal lesions. Endod Dent Traumatol. 1988; 4:49–54. PMID: 3251755.

5. Dahl JE, Frangou-Polyzois MJ, Polyzois GL. In vitro biocompatibility of denture relining materials. Gerodontology. 2006; 23:17–22. PMID: 16433637.
6. Miletić I, Devcić N, Anić I, Borcić J, Karlović Z, Osmak M. The cytotoxicity of Roeko Seal and AH plus compared during different setting periods. J Endod. 2005; 31:307–309. PMID: 15793391.
7. Al-Nazhan S, Spangberg L. Morphological cell changes due to chemical toxicity of a dental material: an electron microscopic study on human periodontal ligament fibroblasts and L929 cells. J Endod. 1990; 16:129–134. PMID: 2388028.

8. ISO-Standards ISO 10993. Biological compatibility of medical devices. Test for cytotoxicity: in vitro methods. part 5. International Organization for Standardization;2009. cited 2013 July 9. https://law.resource.org/pub/ie/ibr/is.en.iso.10993.5.2009.html.
9. Leonardo RT, Consolaro A, Carlos IZ, Leonardo MR. Evaluation of cell culture cytotoxicity of five root canal sealers. J Endod. 2000; 26:328–330. PMID: 11199748.

10. Huang FM, Hsieh YS, Tai KW, Chou MY, Chang YC. Induction of c-fos and c-jun protooncogenes expression by formaldehyde-releasing and epoxy resin-based root-canal sealers in human osteoblastic cells. J Biomed Mater Res. 2002; 59:460–465. PMID: 11774303.

11. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63. PMID: 6606682.

12. Oztan MD, Yilmaz S, Kalayci A, Zaimoğlu A. A comparison of the in vitro cytotoxicity of two root canal sealers. J Oral Rehabil. 2003; 30:426–429. PMID: 12631168.
13. Koulaouzidou EA, Papazisis KT, Beltes P, Geromichalos GD, Kortsaris AH. Cytotoxicity of three resin-based root canal sealers: an in vitro evaluation. Endod Dent Traumatol. 1998; 14:182–185. PMID: 9796482.
14. Pinna L, Brackett MG, Lockwood PE, Huffman BP, Mai S, Cotti E, Dettori C, Pashley DH, Tay FR. In vitro cytotoxicity evaluation of a self-adhesive, methacrylate resin-based root canal sealer. J Endod. 2008; 34:1085–1088. PMID: 18718370.
15. Osorio RM, Hefti A, Vertucci FJ, Shawley AL. Cytotoxicity of endodontic materials. J Endod. 1998; 24:91–96. PMID: 9641138.

16. Azar NG, Heidari M, Bahrami ZS, Shokri F. In vitro cytotoxicity of a new epoxy resin root canal sealer. J Endod. 2000; 26:462–465. PMID: 11199780.
17. Miletić I, Anić I, Karlović Z, Marsan T, Pezelj-Ribarić S, Osmak M. Cytotoxic effect of four root filling materials. Endod Dent Traumatol. 2000; 16:287–290. PMID: 11202896.

18. Geurtsen W, Leinenbach F, Krage T, Leyhausen G. Cytotoxicity of four root canal sealers in permanent 3T3 cells and primary human periodontal ligament fibroblast cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:592–597. PMID: 9619680.

19. Nakamura H, Sakakibara F, Matsumoto Y, Hirano S, Hayakawa H, Sakai K, Yip M. Study on cytotoxicity of root canal filling materials. J Endod. 1986; 12:156–160. PMID: 3461103.
20. Guigand M, Pellen-Mussi P, Le Goff A, Vulcain JM, Bonnaure-Mallet M. Evaluation of the cytotocompatibility of three endodontic materials. J Endod. 1999; 25:419–423. PMID: 10530242.
21. Gerosa R, Menegazzi G, Borin M, Cavalleri G. Cytotoxicity evaluation of six root canal sealers. J Endod. 1995; 21:446–448. PMID: 8537785.

22. Schwarze T, Fiedler I, Leyhausen G, Geurtsen W. The cellular compatibility of five endodontic sealers during the setting period. J Endod. 2002; 28:784–786. PMID: 12470025.

23. Torneck CD, Moe H, Howley TP. The effect of calcium hydroxide on porcine pulp fibroblasts in vitro. J Endod. 1983; 9:131–136. PMID: 6574198.
24. Swierenga SH, MacManus JP, Whitfield JF. Regulation by calcium of the proliferation of heart cells from young adult rats. In Vitro. 1976; 12:31–36. PMID: 942664.

25. Whitfield JF, MacManus JP, Rixon RH, Boynton AL, Yondale T, Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976; 12:1–18. PMID: 172436.
26. Kim CK, Ryu HW, Chang HS, Lee BD, Min KS, Hong CU. Evaluation of the radiopacity and cytotoxicity of resinous root canal sealers. J Korean Acad Conserv Dent. 2007; 32:419–425.

27. Schwarze T, Leyhausen G, Geursten W. Long-term cytocompatibility of various endodontic sealers using a new root canal model. J Endod. 2002; 28:749–753. PMID: 12470017.

28. Kim HJ, Baek SH, Bae KS. Cytotoxicity and genotoxicity of newly developed calcium phosphate-based root canal sealers. J Korean Acad Conserv Dent. 2006; 31:36–49.

29. Park SY, Lee WC, Lim SS. Cytotoxicity and antibacterial property of new resin-based sealers. J Korean Acad Conserv Dent. 2003; 28:162–168.
30. Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS. An in vitro study of the cytotoxicity of two root canal sealers. J Endod. 2000; 26:228–229. PMID: 11199724.
31. Vajrabhaya L, Sithisarn P. Multilayer and monolayer cell cultures in a cytotoxicity assay of root canal sealers. Int Endod J. 1997; 30:141–144. PMID: 10332248.

32. Spångberg LS, Barbosa SV, Lavigne GD. AH-26 releases formaldehyde. J Endod. 1993; 19:596–598. PMID: 8151253.

33. Kaplan AE, Goldberg F, Artaza LP, de Silvio A, Macchi RL. Disintegration of endodontic cements in water. J Endod. 1997; 23:439–441. PMID: 9587297.

34. Araki K, Suda H, Barbosa SV, Spångberg LS. Reduced cytotoxicity of a root canal sealer through eugenol substitution. J Endod. 1993; 19:554–557. PMID: 8151243.

35. Key JE, Rahemtulla FG, Eleazer PD. Cytotoxicity of a new root canal filling material on human gingival fibroblasts. J Endod. 2006; 32:756–758. PMID: 16861076.

36. Watts A, Paterson RC. Cellular responses in the dental pulp: a review. Int Endod J. 1981; 14:10–19. PMID: 7024136.

37. Wataha JC, Hanks CT, Strawn SE, Fat JC. Cytotoxicity of components of resins and other dental restorative materials. J Oral Rehabil. 1994; 21:453–462. PMID: 7965356.

38. Wiegand C, Hipler UC. Methods for the measurement of cell and tissue compatibility including tissue regeneration processes. GMS Krankenhhyg Interdiszip. 2008; 3:Doc12. PMID: 20204114.
Figure 1
Percentages of cell viability in four experimental groups demonstrated significant interaction effects between sealer and time.
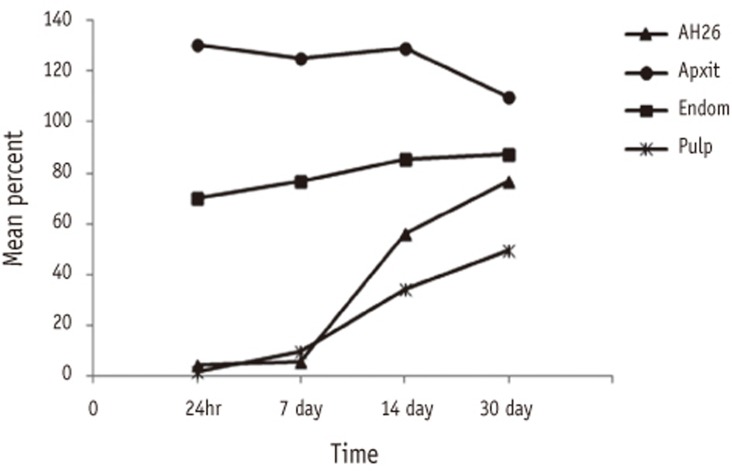
Table 1
Comparison of percentage cell viability (mean ± SD, n = 32) for all sealers at different time periods
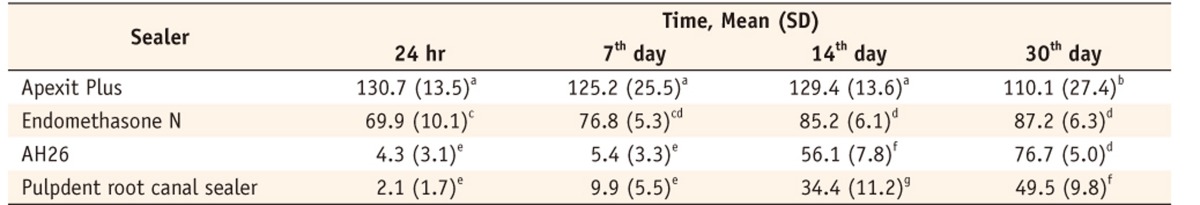
The main effects and the interaction of Sealer and Time were significant (p < 0.001) therefore multiple comparision using Tukey's test was applied in sixteen different groups.
R-squared = 0.932 (Adjusted R-squared = 0.930).
Percentage values with the same superscripts in different groups imply statistical insignificance by Tukey's test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


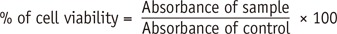
 XML Download
XML Download