Abstract
Objectives
This study evaluated the effect of camphorquinone (CQ)-amine ratio on the C=C double bond conversion of resins with binary and ternary photoinitiation systems.
Materials and Methods
Two monomer mixtures (37.5 Bis-GMA/37.5 Bis-EMA/25 TEGDMA) with binary systems (CQ/DMAEMA in weight ratio, group A [0.5/1.0] and B [1.0/0.5]) and four mixtures with ternary system (CQ/OPPI/DMAEMA, group C [0.1/1.0/0.1], D [0.1/1.0/0.2], E [0.2/1.0/0.1] and F [0.2/1.0/0.2]) were tested: 1 : 2 or 2 : 1 CQ-amine ratio in binary system, while 1 : 1 ratio was added in ternary system. The monomer mixture was cured for 5, 20, 40, and 300 sec with a Demetron 400 curing unit (Demetron). After each exposure time, degree of conversion (DC) was estimated using Fourier transform infrared (FTIR) spectrophotometer (Nicolet 520, Nicolet Instrument Corp.). The results were analyzed by ANOVA followed by Scheffe test, with p = 0.05 as the level of significance.
Results
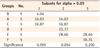
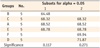
DC (%) was expressed in the order of curing time (5, 20, 40, and 300 sec). Group A (14.63 ± 10.42, 25.23 ± 6.32, 51.62 ± 2.69, 68.52 ± 2.77); Group B (4.04 ± 6.23, 16.56 ± 3.38, 37.95 ± 2.79, 64.48 ± 1.21); Group C (16.87 ± 5.72, 55.47 ± 2.75, 60.83 ± 2.07, 68.32 ± 3.31); Group D (23.77 ± 1.64, 61.05 ± 1.82, 65.13 ± 2.09, 71.87 ± 1.17); Group E (28.66 ± 2.92, 56.68 ± 1.33, 60.66 ± 1.17, 68.78 ± 1.30); Group F (39.74 ± 6.31, 61.07 ± 2.58, 64.22 ± 2.29, 69.94 ± 2.15).
Conclusion
All the monomers with ternary photoinitiation system showed higher DC than the ones with binary system, until 40 sec. Concerning about the effect of CQ-amine ratio on the DC, group A converted into polymer more than group B in binary system. However, there was no significant difference among groups with ternary system, except group C when cured for 5 sec only.
Dental composite resin includes photoinitiation systems that absorb light and take the molecules to excited states. Photopolymerization uses light energy to initiate photochemical and chemical reactions in organic oligomers, to form a new polymeric material by the photo-induced increase of molecular weight by monomer to polymer conversion, as well as crosslinking of developing or preexisting macromolecules. It has been noted that improved photopolymerization is critical for the optimization of mechanical properties, biocompatibility, and color stability of light-activated dental resins.1-3 In addition, optimal (minimal) concentration of camphorquinone (CQ) and amine that causes maximum monomer conversion becomes more important because photoinitiating systems are increasingly being used recently in the field of tissue engineering where the materials are in intimate contact with highly vascularized tissues.4,5
Most photoinitiators used for commercial dental resins consist of two-components (binary system): (i) the photoinitiator (typically a CQ, invented by Dart and Nemcek)6 which can absorb light directly and (ii) a coinitiator (typically an amine) that does not absorb light but interacts with the activated photoinitiator to generate a reactive free radical and initiates polymerization. Absorption of light by CQ typically leads to the creation of two excited states: (i) the 'singlet state', which does not involve reversal of electron spin, and (ii) the 'triplet state', which is the one relevant to free radical formation and which has a very short half-life.7 While in the triplet state, the CQ may interact with an amine molecule and generate an excited complex, the 'exciplex'. Thus, the CQ abstracts a hydrogen atom from the tertiary amine resulting in free radical formation.8
In dental resins formulated with binary photoinitiation system, factors of CQ and amine concentrations, their ratio, the molecular structure of the amine, and the reactivity of the formed radicals to initiate monomer polymerization, all play important roles in the polymerization reaction and, consequently, on the properties of the resultant polymer.9,10 Yoshida and Greener reported the degree of conversion (DC) appeared to reach a maximum at an amine/CQ molar ratio of 3.0.11 Generally, for the same amine/CQ molar ratios, the polymers formulated with 2-(dimethylamino) ethyl methacrylate (DMAEMA), had greater DCs and better physical properties than those formulated with N,N-dimethyl-p-toluidine (DMPT). In a study of Schneider et al., CQ-amine ratios 1 : 1.5 and 1 : 2 showed the best results, regardless of the photoinitiator type (CQ and Phenyl-propanedione [PPD]).12 These results were believed to be probably related to the fact that the reaction between the amine and the tested photoinitiators follows a second-order kinetics, where the rate of polymerization is proportional to the product of concentration (in moles) of two reactants. Due to the differences in the reactants' molar weights (for example, for the 2 : 1 weight ratio, the mixture has a 2.3 : 1 molar ratio for CQ : amine and a 2.6 : 1 molar ratio for the PPD : amine), an excess of amine was present only for the 1 : 2 weight ratio. In a study of Musanje et al., optimal CQ/ethyl-4-dimethylaminobenzoate (EDMAB) concentrations that resulted in maximum Knoop hardness (KHN) were unexpectedly observed at the two regions, one at a low and the other at a high amine concentration.13 The best overlaps (mid-point) of the two regions identified at CQ : EDMAB of 1.44 : 0.42 and 1.05 : 1.65 mol% at the low and high amine concentrations.
These binary CQ-amine photoinitiation systems have their own disadvantages of the toxicity of the used amines and the yellowing of the cured materials caused by oxidation of amine impurities. Furthermore, in CQ-amine photoinitiators as two-component photoinitiating systems, the interaction of the partners is strongly influenced by the viscosity of the medium. Such binary systems also tend to produce a characteristic oxygen-inhibited layer.14 Furthermore, components of binary systems, CQ and amine, have their own problems. Although the visible light photosensitizer CQ, an alpha dicarbonyl that has maximum absorption at 468 nm, is widely used in dental resin and adhesive formulations, it is a solid yellow compound with an unbleachable chromophore group, so that large amounts of CQ in resin formulations lead to an undesirable yellow color, affecting the final aesthetic appearance of the cured material.15 This, in turn, places practical limits on the concentration of CQ and, consequently, limits the degree of polymerization and depth of cure that can be attained.16
CQ has another drawbacks such as low polymerization efficiency and toxicity.17 The photolysis of a diketone leads to the homolytic cleavage of the C-C bond between the two carbonyl groups, resulting in two carbonyl radicals. This radical pair can undergo cage escape to form photodecomposed products. However, the two carbonyl radicals in CQ are structurally connected to each other and the probability of their recombination in CQ is great.18 The consequent low polymerization efficiency of CQ results in relatively low mechanical properties without relatively high CQ concentrations and/or relatively long exposure times, as well as possible toxic effects from unreacted residual monomers.17
Meanwhile, the type of amine directly affects the generation of free radicals. Some amines can actually be used as polymerization inhibitors. The behavior seems to be dependent on many factors, such as the number of methyl groups attached to the nitrogen atom as well as the potential for steric hindrance.19 Above certain limits, any amine co-initiator may act as a retarder because an excess amine may trap initiating radicals by termination reactions.20,21 An additional problem is that excess amine can cause color instability because of the eventual formation of oxidative products.22
Therefore, there has been substantial effort to improve these binary photoinitiation systems by the use of alternative photosensitizers, and various amine reducing agents.18,23-25 In addition, the claim of synergistic effects when two or more initiators are used together has also been reported. Park et al., observed that equal amounts of CQ and PPD produced a higher DC than the sum of the effects of CQ and PPD used separately.16 In recent times, manufacturers included different photoinitiators in the organic matrix to act alone, or synergistically, with CQ. Compounds derived from acylphosphine oxides (monoacylphosphine oxide [MAPO] and bisacylphosphine oxide [BAPO]) and α-diketones (PPD) were used in adhesives and composite resin formulations to improve the polymerization kinetics and lessen the photoyellowing effects.18,26 Unlike CQ, the absorption peak of these compounds is in the near UV region and extends slightly into the visible.16,27 Shin and Rawls reported that dental resin with ternary system of CQ/OPPI/CQ accelerate cure rate, increase conversion, reduce initial color and increase color stability.28 They used the onium compound p-octyloxy-phenyl-phenyl iodonium hexafluoroantimonate (OPPI) as a photoinitiator. It absorbs at 300 - 380 nm, which is outside the range of visible wavelengths, and is therefore colorless. OPPI is a readily soluble white onium salt and can be used as a somewhat hybrid photoinitiator, designed to be able to induce not only cationic polymerization radical but polymerization as well. The onium salt immediately decomposes forming phenyl radicals, which may initiate cure or, abstract a hydrogen from the amine or monomer forming initiating radicals.28 Therefore, it has sufficient energy to initiate the free-radical polymerization.29
There are many uncertainties about the effects of the CQ-amine ratio on the C=C double bond conversion of dental resins formulated with binary and ternary photoinitiation systems. Therefore, this study was carried out in order to evaluate the influence of the CQ-amine ratio on the DC. The hypothesis tested was that the higher the amine ratio, the faster and higher DC in resins with both binary and ternary photoinitiation systems.
Monomer mixture was made by mixing 37.5 wt% 2,2 bis[4-2(2-hydroxy-3-methacroyloxypropoxy)phenyl] propane (Bis-GMA, Esstech, Essington, PA, USA), 37.5 wt% Ethoxylated bisphenol-A dimethacrylate (Bis-EMA, ESSCHEM Inc., Linwood, PA, USA), and 25 wt% triethyleneglycol dimethacrylate (TEGDMA, Esstech).
Two monomer mixtures with binary photoinitiation systems (CQ and DMAEMA, group A, B) and four monomer mixtures with ternary photoinitiation systems (CQ, OPPI and DMAEMA, group C, D, E, F) according to the weight proportion of those components were tested: 1 : 2 or 2 : 1 CQ-amine ratio was tested in binary system, while 1 : 1 ratio was added in ternary system. In all ternary systems, the concentration of OPPI was fixed as 1 wt% based on the result of our preceding study (Table 1).30 Total concentrations of these photoinitiation materials were from 1.2 wt% to 1.5 wt%. Those kinds of works were done under filtered orange light.
Fourier transform infrared (FTIR) absorption spectroscopy is the easiest and simplest method for the determination of the photopolymerization efficiency of dental resins. Therefore, DC was determined using a FTIR spectrophotometer (Nicolet 520, Nicolet Instrument Corp., Madison, WI, USA) in this study.
A small amount of uncured resin monomer was placed between two NaCl disks and the spectrum recorded in transmission with 40 scans at a resolution of 1 cm-1 (baseline). After the IR spectral scan, the monomer mixture was cured between the transparent NaCl disks for 5, 20, 40, 60, and 300 seconds with a Demetron 400 visible light curing unit (Demetron Research Corp., Danbury, CT, USA). The light-intensity of the curing unit was measured prior to the fabrication of each sample set (about 440 mW/cm2) using a Model 100 Optilux radiometer (Kerr, Dansbury, CT, USA).
After each exposure time, the specimens were again scanned for their FTIR spectra. Remaining unconverted double bonds were calculated by comparing the ratio of aliphatic C=C absorption at 1,637 cm-1 or 1,638 cm-1 to aromatic carbon-carbon absorption at 1,608 cm-1 between cured and uncured specimens. Absorption of the aromatic carbon-carbon stretching band remains constant during polymerization and serves as an internal standard. DC was calculated by using the following equation based on the decrease in the intensity band ratios before and after light curing:
DC (%) = [1 - (Rcured / Runcured)] × 100
where R = band height at 1,637 cm-1 or 1,638 cm-1 / band height at 1,608 cm-1.
Rate of conversion at the initial curing period (first 5 seconds) was also calculated by dividing the degree of conversion into 5 seconds in order to verify which group shows the fastest polymerization reaction. All experiments were carried out five times, and the results were analyzed by ANOVA followed by pairwise multiple comparison using Scheffe multiple range comparison test, with p = 0.05 as the level of significance.
Degree of conversion of all groups are shown in Table 2. When monomers were cured for 5 seconds, group B showed the lowest DC (4.04%); group A (14.63%), group C (16.87%), group D (23.77%) and group E (28.66%) were the next (Table 3). DC of group F (39.74%) was the highest one among groups. When monomers were cured for 20 seconds, group B showed the lowest DC (16.56%), the same as when cured for 5 seconds; group A (25.23%) was the next (Table 4). Groups C (55.47%), E (56.68%), D (61.05%), and F (61.07%) showed the higher values without any significant difference among them. When monomers were cured for 40 seconds, same tendency was observed like when they were cured for 20 seconds (Table 5). Group B showed the lowest DC (37.95%), group A (51.62%) was the next. Groups E (60.66%), C (60.83%), F (64.22%) and D (65.13%) showed higher values without any significant difference among them. When monomers were cured for 300 seconds, different tendency was observed (Table 6). Although group B showed the lowest DC (64.48%) and the group D (71.87%) showed the best conversion, the margin was not great. Other groups of C (68.32%), A (68.52%), E (68.78%) and F (69.94%) were the middle.
From the above mentioned data, we can find that the monomers with binary photoinitiation system (groups B and A) did not convert into polymers as much as the ones with ternary photoinitiation system, until 40 seconds curing time. Also, it was revealed that the rate of conversion of monomers with binary system was slower than that of monomers with ternary system (Table 2). Concerning about the effect of CQ-amine ratio on the degree of conversion, monomer with 1 : 2 CQ-amine ratio, group A converted into polymer more than monomer with 2 : 1 CQ-amine ratio, group B in binary system (Tables 2 - 5). However, there was no significant difference among groups with ternary photoinitiation system, except monomer with 1 : 1 CQ-amine ratio, group C when it was cured for 5 seconds only.
Incomplete polymerization can compromise the performance of resin-based dental restoratives. The presence of residual monomer can have a plasticizing effect on the polymer, thereby altering the physical and mechanical properties of the hardened materials.31 In addition, the presence of unreacted monomer can make the polymeric matrix more susceptible to oxidative and hydrolytic degradation reactions, leading to poor durability. It is important, therefore, to evaluate the final degree of conversion of monomer to polymer after polymerization.32 Most commercial dental resins with binary photoinitiation system use CQ as a photoinitiator and amine as a coinitiator, however, their optimal ratio (CQ : amine ratio) for higher DC is controversial.33 Determination of the optimal (minimal) concentration of CQ and amine (ethyl-4-dimethylaminobenzoate, EDMAB) that causes maximum monomer conversion is key to reducing the concentration of residual photoinitiator and amine molecules and minimizing their subsequent potential to leach out into the surrounding tissues and saliva in the case of dental restorative materials.13 Selection of optimal photoinitiator/amine concentration is also critical to materials' formulation, for excessive amounts can compromise materials' properties.13
In this study, higher DC was obtained in group A of monomer with 1 : 2 CQ-amine ratio than group B of monomer with 2 : 1 CQ-amine ratio, in binary system. Although this result is not identical with the Yoshida and Greener's 3.0 amine-CQ molar ratio, it may be coincident with the one of the study of Schneider et al., where 1 : 1.5 and 1 : 2 CQ-amine ratios showed the best results, regardless of the photoinitiator type (CQ and PPD).11,12 It was noted that when the amine concentration is lower than that of CQ, the spontaneous collision of the two substances becomes difficult owing to the small quantity of amine in the reaction. In this case, some molecules of CQ in triplet state return to their fundamental state, reducing the generation of free radicals. When the amine concentration is higher than that of CQ, the production of radicals depends solely on the reactivity of the system, since the collision of molecules will occur because of the excess of amine.34
There was a report that showed hydrophilicity/hydrophobicity of the photoinitiation system might be a controlling factor for the polymerization of monomers.35 The effectiveness of the photoinitiating systems depends on the H-atom donor ability of amines used as coinitiators and the compatibility of initiator components with monomers. CQ is relatively hydrophobic. However, some amines, such as DMAEMA, are hydrophilic and some amines like ethyl 4-dimethylaminobenzoate are relatively hydrophobic. In the presence of water, it is unknown how the hydrophilicity/hydrophobicity of the components in photoinitiator systems would affect polymerization of adhesives.35
Meanwhile, monomers with ternary photoinitiation system showed different conversion pattern from those with binary system in the aspect of the effect of CQ-amine ratio on the DC. There was no significant difference among groups, except monomer with 1 : 1 ratio, group C when it was cured for 5 seconds only. Another 1 : 1 CQ-amine ratio, group F showed the best conversion at all times. The only difference between group C and F was the concentration only. The concentration of CQ and amine in group C was 0.1 wt% each, whereas the concentration in group F was 0.2 wt% each. Therefore, there seems no superior CQ-amine combination in monomers with ternary system.
This kind of DC difference between binary and ternary system can be presumed from the report that the relative concentration of CQ to that of the amine (i.e., CQ : amine ratio) required to cause maximum polymerization of a given polymer system varies depending on the type of amine.11 Thus the CQ to amine ratio required for the maximum polymerization of a given monomer system with a given amine does not apply when another type of amine is used. In addition, because monomers with ternary photoinitiation system have one more component, OPPI compared to those with binary system, so it seems that OPPI may affect the DC of the monomers even if the concentration was fixed into 1.0 wt%. OPPI alone has an absorption maximum around 300 nm with a tail out to 380 nm, which is not in accord with the irradiated wavelengths from the conventional dental curing lamps. However, it was informed that the use of photosensitizers is one of the approaches to extend its absorption to longer wavelength ranges by forming bimolecular photosensitization systems with onium salts although the efficiency of bimolecular systems are usually limited by diffusion, especially in highly viscous polymeric systems. For instance, combined, the borate/OPPI complex exhibits a substantial shift of 50 nm towards the visible light, which extends the tail of the absorbance to about 440 nm (conference proceeding 'De Raaff AM, Marino TL, Neckers DC. Optimized cure efficiency using a fluorone visible light photoinitiator and a novel charge transfer: complex initiating system. In: RAI/TECH Conference. 1996.').
It was reported that the DC of the monomers with ternary photoinitiation system is better than that of monomers with binary system. The majority of commercial dentin resins contain camphorquinone (CQ)/amine pairs as photoinitiating systems. The third component, for example, the iodonium salt (diphenyliodonium hexafluorophosphate, DPIHP) is applied into the above two-component systems.36 DPIHP, which is an electron acceptor, has the following double roles: one is to regenerate the dye molecules (CQ) by replacing inactive, terminating radicals with active, phenyl initiating radicals, and another role is to generate additional active phenyl radicals. Due to its unique abilities, the DPIHP is expected to be an efficient, water compatible initiating component.36 When these components were used in adhesives, such as a dental resin of ternary photoinitiation system (CQ/DMAEMA/DPIHP and CQ/EDMAB/DPIHP).32 They showed higher crosslink density than those formulated with the aliphatic amine and a two component initiator system (binary system). The addition of DPIHP to the two-component photoinitiator systems increased the final degree of conversion, glass transition temperature (Tg), storage modulus, and crosslink density, especially in the presence of water. The enhanced properties observed in the presence of the iodonium salt, DPIHP may be due in part to its ability to generate an active phenyl radical.32 It was also reported that conversion values (less than 70%) of the three-component photoinitiation systems were significantly higher than those containing only the two-component systems (approximately 60%).35 Shin and Rawls also reported that dental resin with ternary system of CQ/OPPI/CQ accelerate cure rate, increase conversion, reduce initial color and increase color stability.28 In this study, the similar result was shown that monomers with ternary photoinitiation system (groups C - F) converted into polymers more than that of ones with binary photoinitiation system, until 40 seconds curing time. Also, it was revealed that the rate of conversion of monomers with ternary system was faster than that of monomers with binary system.
There was another try to make it possible without the addition of amine component. Moszner et al., used benzoyltrimethylgermane (BTMGe) and dibenzoyldiethylgermane (DBDEGe).14 These materials show α-cleavage under formation of benzoyl and germyl radicals, which may initiate the free-radical polymerization of present monomers. Accordingly, the germanium compounds BTMGe and DBDEGe can be used as visible light photoinitiators without the addition of an amine as coinitiator. Following conclusion was made that at least BTMGe can also be used as an amine-free visible light photoinitiator in strongly acidic composite formulations. This is of great advantage, because in the CQ-amine photoinitiator, the amine accelerator can be deactivated by the acid-base reaction with strongly acidic monomers.
Total concentrations of photoinitiation system of experimental monomers in this study, were a little bit diverse from 1.2 wt% to 1.5 wt%. There is a possibility this difference may affect the DC of each monomer. In a study of Moin Jan et al., it was recognized that the leachability of the polymerization initiator (CQ, DMPT, and DMAEMA) depended on the degree of conversion of the VL-cured resin.37 Therefore, the optimal concentration of polymerization initiator can be determined from the relation between the degree of conversion and the leached amount of polymerization initiator, which is about 0.6 wt% for CQ/DMPT (1 : 1 in weight) and 0.5 wt% for CQ/DMAEMA (1 : 1 in weight) relative to the UDMA/TEGDMA (1 : 1 in weight) monomer. It is necessary to find out the best combination of photoinitiation systems with variable OPPI concentration under the condition of fixed total concentration in the following study.
Two kinds of thing were not evaluated in this study. One is about resin filler. The amount and the type of filler may affect the DC of the dental composite resin. The other is about the nature of monomer polymerization. Linear or cross-link type polymerization, which was related with the material property, could not be predicted from the way of DC evaluation. Those kinds of factors should also be considered in the following studies.
In summary, it is generally accepted CQ has low DC efficiency and fatal photoyellowing color problem. Therefore, in this study, DC of monomer was estimated, which has ternary photoinitiation system including readily soluble white onium salt, OPPI, in order to overcome the disadvantages of the CQ-amine binary photoinitiation system. The results showed that higher DC was obtained in the monomers with ternary system rather than in ones with binary system.
DC of monomers is believed to be significantly affected by the CQ-amine ratio. This effect was ascertained if it is true in the monomers with binary and ternary photoinitiation system. Higher DC was obtained when the amine concentration is higher than that of CQ in the monomers with binary photoinitiation system. However, that kind of clear tendency could not be seen in the ternary system. Therefore, the hypothesis that the higher the amine ratio, the faster and higher DC in dental resins was partly accepted in the monomers with binary photoinitiation system only.
Figures and Tables
Notes
References
1. Ferracane JL, Greener EH. The effect of resin formulation on the degree of conversion and mechanical properties of dental restorative resins. J Biomed Mater Res. 1986. 20:121–131.

2. Wataha JC, Hanks CT, Strawn SE, Fat JC. Cytotoxicity of components of resins and other dental restorative materials. J Oral Rehabil. 1994. 21:453–462.

3. Imazato S, Tarumi H, Kobayashi K, Hiraguri H, Oda K, Tsuchitani Y. Relationship between the degree of conversion and internal discoloration of light-activated composite. Dent Mater J. 1995. 14:23–30.

4. Liu VA, Bhatia SN. Three-dimensional photopatterning of hydrogels containing living cells. Biomed Microdev. 2002. 4:257–266.
5. Fisher JP, Dean D, Mikos AG. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly (propylene fumarate) biomaterials. Biomaterials. 2002. 23:4333–4343.

6. Dart EC, Nemcek J. Photopolymerisable composition. Great Britain Patent Specification. 1408265. 1971. Japanese Patent No. Toku-Kou-Sho 54-10986 (1979).
7. Tsai L, Charney E. The triplet states of alpha-dicarbonyls. Camphorquinone. J Phys Chem. 1969. 73:2462–2463.
8. Stansbury JW. Curing dental resins and composites by photopolymerization. J Esthet Dent. 2000. 12:300–308.

9. Jakubiak J, Allonas X, Fouassier JP, Sionkowska A, Andrzejewska E, Linden LA, Rabek JF. Camphorquinone-amines photoinitating systems for the initiation of free radical polymerization. Polymer. 2003. 44:5219–5226.

10. Davidenko N, Garcia O, Sastre R. Photopolymerization kinetics of dimethacrylate-based light-cured dental resins. J Appl Polym Sci. 2005. 97:1016–1023.

11. Yoshida K, Greener EH. Effects of two amine reducing agents on the degree of conversion and physical properties of an unfilled light-cured resin. Dent Mater. 1993. 9:246–251.

12. Schneider LF, Cavalcante LM, Consani S, Ferracane JL. Effect of co-initiator ratio on the polymer properties of experimental resin composites formulated with camphorquinone and phenyl-propanedione. Dent Mater. 2009. 25:369–375.

13. Musanje L, Ferracane JL, Sakaguchi RL. Determination of the optimal photoinitiator concentration in dental composites based on essential material properties. Dent Mater. 2009. 25:994–1000.

14. Moszner N, Fischer UK, Ganster B, Liska R, Rheinberger V. Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent Mater. 2008. 24:901–907.

15. Rueggeberg FA, Ergle JW, Lockwood PE. Effect of photoinitiator level on properties of a light-cured and post-cure heated model resin system. Dent Mater. 1997. 13:360–364.

16. Park YJ, Chae KH, Rawls HR. Development of a new photoinitiation system for dental light-cure composite resins. Dent Mater. 1999. 15:120–127.

17. Fujisawa S, Kadoma Y, Masuhara E. Effects of photoinitiators for the visible-light resin system on hemolysis of dog erythrocytes and lipid peroxidation of their components. J Dent Res. 1986. 65:1186–1190.

18. Sun GJ, Chae KH. Properties of 2,3-butanedione and 1-phenyl-1,2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer. 2000. 41:6205–6212.

19. Jakubiak J, Wrzyszczyński A, Lindén LÅ, Rabek JF. The role of amines in the camphorquinone photoinitiated polymerization of multifunctional monomer. J Macromol Sci A. 2007. 44:239–242.

20. Davidenko N, Garcia O, Sastre R. Photopolymerization kinetics of dimethacrylate-based light-cured dental resins. J Appl Polym Sci. 2005. 97:1016–1023.

21. Jakubiak J, Sionkowska A, Lindén LÅ, Rabek JF. Isothermal photo differential scanning calorimetry: cross-linking polymerization of multifunctional monomers in presence of visible light photoinitiators. J Therm Anal Calor. 2001. 65:435–443.
22. Bowen RL, Argentar H. Tertiary aromatic amine accelerators with molecular weights above 400. J Dent Res. 1972. 51:473–482.

23. Ogunyinka A, Palin WM, Shortall AC, Marquis PM. Photoinitiation chemistry affects light transmission and degree of conversion of curing experimental dental resin composites. Dent Mater. 2007. 23:807–813.

24. Cohen SG, Chao HM. Photoreduction of aromatic ketones by amine. Studies of quantum yields and mechanism. J Am Chem Soc. 1968. 90:165–173.

25. Antonucci JM, Venz S. Tertiary amine salts and complexes as chemical and photochemical accelerators. J Dent Res. 1987. 66:128. Abstract #170.
26. Tanoue N, Matsumura H, Atsuta M. The influence of ultraviolet radiation intensity on curing depth of photoactivated composite veneering materials. J Oral Rehab. 1998. 25:770–775.
27. Allen NS. Photoinitiators for UV and visible curing of coatings: mechanism and properties. J Photochem Photobiol A Chem. 1996. 100:101–107.

28. Shin DH, Rawls HR. Degree of conversion and color stability of the light curing resin with new photoinitiator systems. Dent Mater. 2009. 25:1030–1038.

29. He JH, Mendoza VS. Synthesis and study of a novel hybrid UV photoinitiator: p-benzoyldiphenyliodonium hexafluorophosphate (PhCOPhl + PhPF). J Polym Sci A: Polym Chem. 1996. 34:2809–2816.

30. Kim CG, Moon HJ, Shin DH. Optimal combination of 3-component photoinitiation system to increase the degree of conversion of resin monomers. J Korean Acad Conserv Dent. 2011. 36:313–323.

31. Vallo CI. Flexural strength distribution of a PMMA-based bone cement. J Biomed Mater Res. 2002. 63:226–236.

32. Park J, Ye Q, Topp EM, Misra A, Kieweg SL, Spencer P. Effect of photoinitiator system and water content on dynamic mechanical properties of a light-cured bisGMA/HEMA dental resin. J Biomed Mater Res A. 2010. 93:1245–1251.

33. Brandt WC, Schneider LF, Frollini E, Correr-Sobrinho L, Sinhoreti MA. Effect of different photo-initiators and light curing units on degree of conversion of composites. Braz Oral Res. 2010. 24:263–270.

34. Cook WD. Photopolymerization kinetics of dimethacrylates using the camphorquinone/amine initiator system. Polymer. 1992. 33:600–609.

35. Guo X, Wang Y, Spencer P, Ye Q, Yao X. Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent Mater. 2008. 24:824–831.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download