Abstract
Objectives
This study evaluated the effect of 2% chlorhexidine digluconate (CHX) with different application times on microtensile bonds strength (MTBS) to dentin in class I cavities and intended to search for ideal application time for a simplified bonding protocol.
Materials and Methods
Flat dentinal surfaces with class I cavities (4 mm × 4 mm × 2 mm) in 40 molar teeth were bonded with etch-and-rinse adhesive system, Adper Single Bond 2 (3M ESPE) after: (1) etching only as a control group; (2) etching + CHX 5 sec + rinsing; (3) etching + CHX 15 sec + rinsing; (4) etching + CHX 30 sec + rinsing; and (5) etching + CHX 60 sec + rinsing. Resin composite was built-up with Z-250 (3M ESPE) using a bulk method and polymerized for 40 sec. For each condition, half of the specimens were immediately submitted to MTBS test and the rest of them were assigned to thermocycling of 10,000 cycles between 5℃ and 55℃ before testing. The data were analyzed using two-way ANOVA, at a significance level of 95%.
Results
There was no significant difference in bond strength between CHX pre-treated group and control group at the immediate testing period. After thermocycling, all groups showed reduced bond strength irrespective of the CHX use. However, groups treated with CHX maintained significantly higher MTBS than control group (p < 0.05). In addition, CHX application time did not have any significant influence on the bond strength among groups treated with CHX.
Despite successful immediate bonding, the longevity of the adhesive interface remains questionable due to physical and chemical factors challenging the adhesive interface.1,2 The loss of bond strength has been attributed primarily to degradation of the hybrid layer at the dentin-adhesive interface and deterioration of the dentin collagen fibrils.3-8 Although the current strategies of incorporating ionic and hydrophilic resinous components into total-etch and self-etch adhesives arise from the need to bond to an intrinsically wet substrate, these strategies have created potentially unstable resin matrices that slowly degrade via water sorption. Moreover, temperature change, chewing loads and chemical attacks by acids and enzymes in the oral cavity have represented a significant challenge to tooth-composite bond survival for some time.9
Bonding is created by impregnating the dentin substrate with blends of resin monomers, and the stability of the bonded interface relies on the creation of a compact and homogenous hybrid layer. In the etch-and rinse strategy, after preliminary etching to demineralize the substrate, resin monomers impregnate the porous etched substrate and thus stable bonds may be achieved if the etched substrate is fully infiltrated by the adhesive.10-12
However, a decreasing gradient of resin monomer diffusion within acid-etched dentinresults in incompletely infiltrated zones along the bottom of hybrid layers that contain denuded collagen fibrils.13,14 These zones are micromorphologically seen as different modes of nanoleakage within the hybrid layers, they are sites that are susceptible to degradation.3,7,15 It has been speculated that this decreasing gradient of resin monomer diffusion within acid-etched dentin and the resin elution from hydrolytically unstable polymeric hydrogels within the hybrid layers leave collagen fibrils unprotected and also vulnerable to degradation by endogenous metaloproteinases in a way that is similar to what occurs in caries progression and periodontal disease.3,16-19
Matrix metalloproteinases (MMPs) are a family of host-derived proteolytic enzymes that are capable of degrading the organic matrix of demineralized dentin.16 Human dentin contains collagenase (MMP-8) and gelatinases (MMP-2 and -9), among others.17 This dentin collagenolytic and gelatinolytic activity can be suppressed by protease inhibitor, indicating that MMPs inhibition could be beneficial in preservation of the hybrid layers.8 Therefore, one might consider that preventing the degradation of incomplete resin-infiltrated collagen fibrils by MMPs is an important issue to be investigated, since this could be the key to the increased durability of restorations that involve bonding to dentin substrate.
Both in vitro and in vivo studies have shown that 2% chlorhexidine digluconate (CHX), applied for 60 seconds on demineralized dentin, postpones the resin-dentin degradation of adhesive interfaces, when compared with interfaces to which no CHX is applied.20-23 CHX is widely used as an antimicrobial agent and possesses a broad spectrum of activity against oral bacteria.24,25 Thus, apart from being a commonly known disinfectant, it was shown that the dentinal collagenolytic activity can be strongly reduced by the use of CHX, a potent MMP inhibitor.8 CHX also prevents or minimizes the auto-degradation of exposed collagen fibrils within incompletely-formed hybrid layer, thereby, contributing to the long-term stability of the hybrid layer and bond strength.20 In addition, CHX may also be a useful complementary method to other techniques of proven efficacy for rehydrating dried mineralized dentin and, therefore, preserving the humidity necessary for keeping the collagen network expanded.9 Despite these advantages, the use of 2% CHX for 60 seconds demands more chair-time during the adhesive procedure and this contrasts with the clinicians' needs for simplification.26
In a recent investigation, when 2% CHX containing phosphoric acid was applied for 15 seconds the durability of the resin-dentin bonds was preserved. This seems to indicate that even a short period of CHX application in contact with the demineralized dentin appears to be sufficient to inhibit the action of specific host-derived proteinases.27 Therefore, the ideal situation would be suggested to apply CHX for a short period of time, as part of the strategy to simplify the bonding protocol.
The aim of this in vitro study was to evaluate the effect of CHX with different application times on microtensile bonds strength (MTBS) to dentin in class I cavities and with a puopose of searching for ideal period of application time to simplify bonding protocol. The tested hypotheses are that 1) CHX does not cause a detrimental effect on MTBS to dentin; 2) CHX preserves the durability of the resin-dentin bonds even after thermocycling; 3) CHX application time does not influence MTBS after thermocycling.
Forty freshly extracted caries-free molars, which had been stored in 0.1% thymol solution were used in this study. The occlusal enamel was ground flat using a model trimmer (Model Trimmer, Sejong, Korea) under running water, then abraded with wet 600-grit silicon carbide abrasive paper to expose a flat dentin surface that permitted placing the cavity margins in dentin. Class I cavities (4 mm × 4 mm × 2 mm) were prepared in dentin using a diamond bur in a high-speed hand-piece with copious air-water spray (Figure 1). Specimens that showed visible pulp exposure were excluded from the study.
All the cavities were submitted to the bonding protocols using two-step etch-and-rinse (Adper Single Bond 2, 3M ESPE, St. Paul, MN, USA) adhesive system and a light-curing composite (Z-250, 3M ESPE). The materials used in this study and their compositions are listed in Table 1. All the materials were handled according to the manufacturer's instructions.
Forty teeth were randomly divided into 5 groups of 8 teeth each. Table 2 shows the experimental groups with their respective modalities.
In group 1 (Control group), the dentin surfaces were acid etched with 37% phosphoric acid and washed with water spray for 10 seconds and gently dried for 10 seconds and the adhesive was applied.
In groups 2, 3, 4, and 5 the dentin surfaces were acid etched with 37% phosphoric acid, rinsed off, air-dried as the same manner as group 1, then rewetted with Consepsis (Ultradent Products Inc., South Jordan, UT, USA), a 2% CHX cavity disinfectant. Rewetting procedures were performed for 5, 15, 30, and 60 seconds, respectively. Afterwards, the adhesive systems were applied according to the manufacturer's instructions.
Following the pre-treatment sequences of the individual groups, the cavities were filled with Z-250 (3M ESPE) resin composite using a bulk method and additional 2 mm thick of resin composite were built up and were polymerized for 40 seconds. Resin composite was then built up for the MTBS.
Half of the specimens for each group were stored in water for 24 hours, and the other half of the specimens were then subjected to thermocycling of 10,000 cycles with temperature changing from 5℃ to 55℃, with a dwelling time for 15 seconds each and interval time for 10 seconds.
After 24 hours of storage in 37℃ water, the restored specimens were serially sectioned into 1 mm thick dentin-resin slabs (Figure 2a), rotated 90°, and then sectioned again to obtain resin-dentin sticks from the cavity floor with a rectangular cross-sectional area of approximately 1 mm2 using Accutom 50 (Struers., Compenhagen, Denmark). Two to four sticks were obtained from each restoration (Figure 2b) and total fifteen sticks were used in each group. The bonded surface area was calculated before each test by measuring the narrowest portion with a digital caliper (Mitutoyo, Tokyo, Japan) to the nearest 0.01 mm. The ends of the sticks were glued to a testing machine (EZ Test, Shimadzu Co., Kyoto, Japan) using cyanoacrylate glue (Superglue, Henkel Loctite Ireland, Dublin, Ireland), and subjected to a MTBS testing at a crosshead speed of 1 mm/min (Figure 2c).
The results of the MTBS values are summarized in Table 2. The factor of thermocycling (p < 0.001) showed a significant effect, but the effect of CHX treatment time (p = 0.863) and their interaction (p = 0.401) was not significantly different (Table 3). Therefore, the effect of CHX and effect of CHX application time in MTBS were analyzed separately.
In this study, there was no significant difference of bond strength between CHX pre-treated group and control group at the immediate testing period. After 10,000 cycles of thermocycling, all groups showed reduced bond strength irrespective of the CHX use. However, groups treated with CHX resulted in a smaller reduction in bond strength than control group (p < 0.05).
When the bond strengths were compared on the basis of each test time, CHX application time did not have any significant difference among groups treated with CHX.
In the present study, CHX did not affect the bond strength of the specimens immediately subjected to MTBS test. This was also observed in previous in vivo study using the etch-and-rinse adhesive Single bond.23 CHX is a widely used antimicrobial agent that possesses a broad spectrum of activity against oral bacteria and has a low toxicity. This is why several studies have proposed the use of CHX for cavity disinfection before placement of restoration. The ideal timing for CHX application has been questioned, leading some authors to use it to treat the prepared cavity before or after acid etching.9,28,29 Authors who apply CHX after acid etching believe that this procedure can increase the wetting of dentin for primers, as CHX solution produces some debris on the surface and within the tubules of etched dentin.30 Certain CHX properties, such as a strong positive ionic charge, readily binding to phosphate groups, a strong affinity to the tooth surface that is increased by acid etching and finally capacity to increase the surface-free energy of enamel and perhaps dentin, are probably responsible for the good resin-dentin bond strengths obtained when CHX is applied after acid etching.30,31 According to the results of the current study compared to those published in the literature, the application of CHX produces no detrimental effect on dentin adhesion in immediate time.9,28,29
Several in vitro and in vivo studies of the same working group validated the concept that CHX may prevent exposed collagen within dentin bonds from degradation, thereby improving its longevity.20-23 The results of the current study corroborated with such findings. When 2% CHX solution was used, after 10,000 cycles of thermocycling, some degree of reduction in bond strength was observed, but that was less than that of control group. Contrary findings were observed in the control groups, which demonstrated reductions of approximately 40%, in terms of bond strength values. Therefore, the first hypothesis, CHX does not cause a detrimental effect on MTBS to dentin and preserve the durability of the resin-dentin bonds even after thermocycling, was partially accepted.
Interestingly, the good performance of CHX in the preservation of the dentin bonds over time was independent of its application time. The high substantibity of CHX may explain why the application time did not have a significant effect. CHX is one of the most commonly used antimicrobial agents because it retains a therapeutic effect for prolonged period of time. The substantivity of CHX is related to the release of positively charged molecules from CHX-treated surfaces and its ability to adsorb onto surfaces of the oral cavity.30,31 Theoretically, this can also occur in the demineralized exposed collagen fibrils, and is the explanation for the bonds being preserved after long-term water exposure. Moreover, one may suggest that CHX is likely to bind to collagen fibrils at a very fast rate, and thus even short periods of time, seem to be sufficient to guarantee such binding. In recent investigation, the application of a 2% CHX solution containing phosphoric acid for 15 seconds was sufficient to maintain the stability of the resin-dentin bond after 6 months of water storage.27 This suggests only a few seconds of contact with CHX might be sufficient to inhibit the MMP activity and corroborate the findings of the present investigation. Therefore, the second hypothesis, CHX application time could not influence the bond strength, was totally accepted.
It is well known that the stress from polymerization shrinkage is influenced by restorative techniques, modulus of resin elasticity, polymerization rate, and cavity configuration or 'C-factor' which is defined as the quotient between bonded and unbonded resin composite surface area.32 In this study, MTBS value on the whole was lower than previous study which evaluated the effect of CHX on the durability of resin-dentin bonds.9,27,33 Logercio reported 43.5 MPa for 15 seconds application, 41.2 MPa a for 60 seconds application in immediate bonding and 40.1 MPa for 15 seconds, 37.6 MPa for 60 seconds after water storage for 6 months.33 The concept of the cavity configuration factor appears to be an appropriate explanation for this discrepancy. Other previous studies, they evaluated the MTBS on flat superficial dentin. However, Class I cavity was prepared after flat enamel surface grinding with the depth of 2 mm in this study. When light-cured resin composite in placed into a box-like cavity by means of a bulk filling technique, the competition between polymerization shrinkage and adhesion between the resin and dentin is maximized and weakens the bond strength. Another factor which can also reduce the bond strength is dentin depth. Deep dentin has a higher water content than does superficial dentin due to the larger diameter and number of tubules per unit area. This water may dilute the organic solvents of some bonding systems, causing monomers to leave the soluble phase and form resin globules in water.34 If components of the free-radical-generating system are soluble in water, they may create a partition between the organic solvent of the bonding system and water in such a manner that interferes with conversion of monomers to polymers. These two factors explain lower bond strength in this study compare to the previous studies of etch-and-rinse system.9,27,33
One cannot ignore the fact that despite the advantages of using 2% CHX, after acid etching includes another bonding step during the restorative procedure and this works against the clinician's need for simplification.26 In this study, the application time of CHX was reduced (5 seconds) and this reduction in application time did not jeopardize the benefits of CHX in the preservation of the dentin bonds. Therefore, the results of the present investigation suggest that 2% CHX, applied for 5 seconds, is sufficient to preserve dentin bonds for at least 10,000 cycles under the laboratory conditions of this study. However, further in vivo studies are needed to clarify whether the use of the 2% CHX solution for short period of time is able to preserve resin-dentin bonds after long-term function.
CHX pre-treatment did not make any harmful effect on in vitro dentin bond strength before thermocycling, compared to the control group. All groups showed some of bond strength reduction after 10,000 cycles of thermocycling, but that amount of reduction was greater in control group (p < 0.05) than in groups treated with CHX. However, different application time of CHX did not make any difference in dentin bond strength among groups.
In summary, a short time (5 seconds) application of 2% CHX after etching with 37% phosphoric acid seems to be sufficient to preserve MTBS over 10,000 cycles of thermocycling.
Figures and Tables
Figure 1
Preparation of bonding substrate. (a) Extracted human tooth before preparation, (b) After enamel removal and cavity preparation (4 mm × 4 mm × 2 mm).
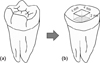
Figure 2
Specimen preparation for microtensile bond strength test. (a) Bonding substrate were restored with adhesive and composite, and excess composite was built up on the outer surface of the restoration; (b) Resin-dentin sticks with a rectangular cross-sectional area of approximately 1 mm × 1 mm; (c) Microtensile bond strength test at a crosshead speed of 1 mm/min.
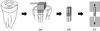
References
1. De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005. 84:118–132.

2. Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008. 24:90–101.

3. Wang Y, Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J Dent Res. 2003. 82:141–145.

4. Wang Y, Spencer P. Continuing etching of an all-in-one adhesive in wet dentin tubules. J Dent Res. 2005. 84:350–354.

5. Yiu CK, King NM, Pashley DH, Suh BI, Carvalho RM, Carrilho MR, Tay FR. Effect of resin hydrophilicity and water storage on resin strength. Biomaterials. 2004. 25:5789–5796.

6. Carrilho MR, Tay FR, Pashley DH, Tjäderhane L, Carvalho RM. Mechanical stability of resin-dentin bond components. Dent Mater. 2005. 21:232–241.

7. Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resin-dentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials. 2003. 24:3795–3803.

8. Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004. 83:216–221.

9. Soares CJ, Pereira CA, Pereira JC, Santana FR, do Prado CJ. Effect of chlorhexidine application on microtensile bond strength to dentin. Oper Dent. 2008. 33:183–188.

10. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982. 16:265–273.

11. Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, Van Landuyt K, Lambrechts P, Vanherle G. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003. 28:215–235.
12. Yoshida Y, Van Meerbeek B, Snauwaert J, Hellemans L, Lambrechts P, Vanherle G, Wakasa K, Pashley DH. A novel approach to AFM characterization of adhesive tooth-biomaterial interfaces. J Biomed Mater Res. 1999. 47:85–90.

13. Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002. 62:447–456.

14. Hashimoto M, Ohno H, Kaga M, Sano H, Endo K, Oguchi H. The extent to which resin can infiltrate dentin by acetone-based adhesives. J Dent Res. 2002. 81:74–78.

15. Tay FR, Pashley DH, Yoshiyama M. Two modes of nanoleakage expression in single-step adhesives. J Dent Res. 2002. 81:472–476.

16. Tjäderhane L, Larjava H, Sorsa T, Uitto VJ, Larmas M, Salo T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J Dent Res. 1998. 77:1622–1629.

17. Sulkala M, Larma M, Sorsa T, Salo T, Tjäderhane L. The localization of matrix metalloproteinases-20 (MMP-20, enamelysin) in mature human teeth. J Dent Res. 2002. 81:603–607.

18. Van Strijp AJ, Jansen DC, DeGroot J, Ten Cate JM, Everts V. Host-derived proteinases and degradation of dentine collagen in situ. Caries Res. 2003. 37:58–65.

19. Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodont Res. 1995. 30:23–33.

20. Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005. 84:741–746.

21. Brackett WW, Tay FR, Brackett MG, Dib A, Sword RJ, Pashely DH. The effect of chlorhexidine on dentin hybrid layers in vivo. Oper dent. 2007. 32:107–111.
22. Carrilho MR, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, Pashley DH, Tjäderhane L. Chlorhexidine preserves dentin bond in vitro. J Dent Res. 2007. 86:90–94.
23. Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley D. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007. 86:529–533.

24. Stanley A, Wilson M, Newman HN. The in vitro effects of chlorhexidine on subgingival plaque bacteria. J Clin Periodontol. 1989. 16:259–264.

25. Bok YB, Lee DY, Lee CY, Kim KN, Kum KY. The sustaining effect of three polymers on the release of chlorhexidine from a controlled release drug device for root canal disinfection. J Korean Acad Conserv Dent. 2004. 29:548–554.

26. Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003. 69:726–731.
27. Stanislawczuk R, Amaral RC, Zander-Grande C, Gagler D, Reis A, Loguercio AD. Chlorhexidine-containing acid conditioner preserves the longevity of resin-dentin bonds. Oper Dent. 2009. 34:481–490.

28. Chang YE, Shin DH. Effect of chlorhexidine application methods on microtensile bond strength to dentin in Class I cavities. Oper Dent. 2010. 35:618–623.

29. de Castro FL, de Andrade MF, Duarte Júnior SL, Vaz LG, Ahid FJ. Effect of 2% chlorhexidine on microtensile bond strength of composite to dentin. J Adhes Dent. 2003. 5:129–138.
30. Perdigao J, Denehy GE, Swift EJ Jr. Effects of chlorhexidine on dentin surfaces and shear bond strengths. Am J Dent. 1994. 7:81–84.
31. Fardal O, Turnbull RS. A review of the literature on use of chlorhexidine in dentistry. J Am Dent Assoc. 1986. 112:863–869.

32. Kim Y, Park JW, Lee CY, Song YJ, Seo DK, Roh BD. The influence of cavity configuration on the microtensile bond strength between composite resin and dentin. J Korean Acad Conserv Dent. 2008. 33:472–480.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download