Abstract
Objectives
The aim was to confirm the stem cell-like properties of the dental pulp stromal cells and to evaluate the morphologic changes during in vitro chondrogenesis.
Materials and Methods
Stromal cells were outgrown from the dental pulp tissue of the premolars. Surface markers were investigated and cell proliferation rate was compared to other mesenchymal stem cells. Multipotency of the pulp cells was confirmed by inducing osteogenesis, adipogenesis and chondrogenesis. The morphologic changes in the chondrogenic pellet during the 21 day of induction were evaluated under light microscope and transmission electron microscope. TUNEL assay was used to evaluate apoptosis within the chondrogenic pellets.
Results
Pulp cells were CD90, 105 positive and CD31, 34 negative. They showed similar proliferation rate to other stem cells. Pulp cells differentiated to osteogenic, adipogenic and chondrogenic tissues. During chondrogenesis, 3-dimensional pellet was created with multi-layers, hypertrophic chondrocyte-like cells and cartilage-like extracellular matrix. However, cell morphology became irregular and apoptotic cells were increased after 7 day of chondrogenic induction.
Figures and Tables
Figure 1
Characterization of the dental pulp stromal cells.
(a) Fibroblast-like cells were collected from dental pulp tissue; (b) Pulp cells were positive for mesenchymal cell surface markers CD 90 and 105; (c) Proliferation rates of pulp cells from premolar (DP4), and 3rd molar (DP8) were similar to those of bone marrow mesenchymal stem cell (BMMSC) and adipogenic stem cell (ADSC).

Figure 2
Multipotency of the pulp stromal cells.
(a) Osteogenesis; (b) Adiopogenesis; (c) Chondrogenesis after 14 days of induction. Scale bar = 50 mm
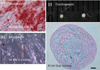
Figure 3
Morphologic evaluation of the chondrogenic pellets engineered from pulp cells.
HE (a, b, c, d) and Alcian blue (e, f, g, h) staining at 3, 7, 14 and 21 days of chondrogenic induction. Scale bar = 50 µm. TEM (i, j, k, l) images of the cells within the pellets at 3, 7, 14 and 21 days of chondrogenic induction. White arrows, nucleolus; black arrow, extracellular matrix fibers, scale bar = 5 µm. HE, Hematoxylin-eosin; TEM, transmission eletron microscope.
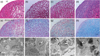
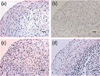
Figure 4
Evaluation of apoptotic cells within the chondrogenic pellets.
(a) At day 3, few Tunel positive cells were localized only within the superficial layer, but as induction period increased Tunel positive cells were scattered throughout the pellet; (b) at day 7; (c) at day 14; (d) at day 21. Scale bar = 50 µm
References
1. Okeson JP. Management of temporomandibular disorder 5th edition. 2003. Philadelphia: Elsevier;15–22.
2. Arnett GW, Milam SB, Gottesman L. Progressive mandibular retrusion-idiopathic condylar resorption. Part II. Am J Orthod Dentofacial Orthop. 1996. 110:117–127.

3. Arnett GW, Milam SB, Gottesman L. Progressive mandibular retrusion-idiopathic condylar resorption. Part I. Am J Orthod Dentofacial Orthop. 1996. 110:8–15.

4. Wolford LM, Cardenas L. Idiopathic condylar resorption: diagnosis, treatment protocol, and outcomes. Am J Orthod Dentofacial Orthop. 1999. 116:667–677.

5. Crawford JG, Stoelinga PJ, Blijdorp PA, Brouns JJ. Stability after reoperation for progressive condylar resorption after orthognathic surgery: report of seven cases. J Oral Maxillofac Surg. 1994. 52:460–466.

6. De Clercq CA, Neyt LF, Mommaerts MY, Abeloos JV, De Mot BM. Condylar resorption in orthognathic surgery: a retrospective study. Int J Adult Orthodon Orthognath Surg. 1994. 9:233–240.
7. Merkx MA, Van Damme PA. Condylar resorption after orthognathic surgery. Evaluation of treatment in 8 patients. J Craniomaxillofac Surg. 1994. 22:53–58.
8. McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, Steadman JR. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011. 27:1552–1561.

9. Meyerrose T, Olson S, Pontow S, Kalomoiris S, Jung Y, Annett G, Bauer G, Nolta JA. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010. 62:1167–1174.

10. van Buul GM, Kotek G, Wielopolski PA, Farrell E, Bos PK, Weinans H, Grohnert AU, Jahr H, Verhaar JA, Krestin GP, van Osch GJ, Bernsen MR. Clinically translatable cell tracking and quantification by MRI in cartilage repair using superparamagnetic iron oxides. PLoS One. 2011. 6:e17001.

11. Zhang J, Pan T, Im HJ, Fu FH, Wang JH. Differential properties of human ACL and MCL stem cells may be responsible for their differential healing capacity. BMC Med. 2011. 9:68.

12. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000. 97:13625–13630.
13. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004. 364:149–155.

14. Huang AH, Chen YK, Chan AW, Shieh TY, Lin LM. Isolation and characterization of human dental pulp stem/stromal cells from nonextracted crown-fractured teeth requiring root canal therapy. J Endod. 2009. 35:673–681.

15. Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N, Franchina M, Grossi A, Bagnara GP. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005. 80:836–842.

16. Struys T, Moreels M, Martens W, Donders R, Wolfs E, Lambrichts I. Ultrastructural and immunocytochemical analysis of multilineage differentiated human dental pulp- and umbilical cord-derived mesenchymal stem cells. Cells Tissues Organs. 2011. 193:366–378.

17. Ichinose S, Muneta T, Koga H, Segawa Y, Tagami M, Tsuji K, Sekiya I. Morphological differences during in vitro chondrogenesis of bone marrow-, synovium-MSCs, and chondrocytes. Lab Invest. 2010. 90:210–221.

18. Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006. 324:225–236.

19. Song SY, Jung JE, Jeon YR, Tark KC, Lew DH. Determination of adipose-derived stem cell application on photo-aged fibroblasts, based on paracrine function. Cytotherapy. 2011. 13:378–384.

20. Kim NR, Lee DH, Ahn SJ, Lee IS, Yang HC. The differentiation-inducing effect of conditioned media obtained from dental pulp cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. 107:e54–e59.

21. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.

22. Ahrens PB, Solursh M, Reiter RS. Stage-related capacity for limb chondrogenesis in cell culture. Dev Biol. 1977. 60:69–82.

23. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003. 100:5807–5812.

24. Nam H, Lee G. Identification of novel epithelial stem cell-like cells in human deciduous dental pulp. Biochem Biophys Res Commun. 2009. 386:135–139.

25. Choi KM, Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, Park JK. Effects of mechanical stimulation on the proliferation of bone marrow-derived human mesenchymal stem cells. Biotechnology and Bioprocess Engineering. 2007. 12:601–609.

26. Han MJ, Seo YK, Yoon HH, Song KY, Park JK. Effect of mechanical tension on the human dental pulp cells. Biotechnology and Bioprocess Engineering. 2008. 13:410–417.

27. Tanaka K, Iwasaki K, Feghali KE, Komaki M, Ishikawa I, Izumi Y. Comparison of characteristics of periodontal ligament cells obtained from outgrowth and enzyme-digested culture methods. Arch Oral Biol. 2011. 56:380–388.

28. Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009. 24:347–366.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download