Abstract
Background:
The present study was attempted to compare enalapril, an angiotensin-converting enzyme inhibitor with losartan an angiotensin II (Ang II) receptor blocker in the inhibitory effects on the secretion of catecholamines (CA) from the perfused model of the rat adrenal gland.
Methods:
The adrenal gland was isolated and perfused with Krebs-bicarbonate. CA was measured directly by using the fluorospectrophotometer.
Results:
Both enalapril and losartan during perfusion into an adrenal vein for 90 minutes inhibited the CA release evoked by acetylcholine (ACh), 1.1-dimethyl-4-phenyl piperazinium (DMPP, a selective Nn agonist), high K+ (a direct membrane-depolarizer), 3-(m-chloro-phenyl-carbamoyl-oxy-2-butynyl–trimethyl ammonium (McN-A-343, a selective M1 agonist), and Ang II in a time-dependent manner. Also, in the presence of enalapril or losartan, the CA release evoked by veratridine (an activator of voltage-dependent Na+ channels), 6-dimethyl-3-nitro-4-(2-trifluoromethyl phenyl)-pyridine-5-carboxylate (BAY-K-8644, an L-type Ca – 2+ channel activator), and cyclopiazonic acid (a cytoplasmic Ca2+-ATPase inhibitor) were significantly reduced. Based on the same concentration of enalapril and losartan, for the CA release evoked by ACh, high K+, DMPP, McN-A-343, Ang II, veratridine, BAY-K-8644, and cyclopiazonic acid, the following rank order of inhibitory potency was obtained: losartan > enalapril. In the simultaneous presence of enalapril and losartan, ACh-evoked CA secretion was more strongly inhibited compared with that of enalapril- or losartan-treated alone.
Conclusions:
Collectively, these results demonstrate that both enalapril and losartan inhibit the CA secretion evoked by activation of both cholinergic and Ang II type-1 receptors stimulation in the perfused rat adrenal medulla. When these two drugs were used in combination, their effects were enhanced, which may also be of clinical benefit. Based on concentration used in this study, the inhibitory effect of losartan on the CA secretion seems to be more potent than that of enalapril.
Go to : 
References
1. Douglas WW, Poisner AM. Stimulation of uptake of calcium-45 in the adrenal gland by acetylcholine. Nature. 1961; 192:1299.

2. Wilson SP, Kirshner N. The acetylcholine receptor of the adrenal medulla. J Neurochem. 1977; 28:687–95.

3. Holz RW, Senter RA, Frye RA. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem. 1982; 39:635–46.
4. Gavras I, Gavras H. Angiotensin II-possible adverse effects on arteries, heart, brain, and kidney: experimental, clinical, and epidemiological evidence. Robertson JI, Nicholls MG, editors. The renin-angiotensin system. London: Gower Medical Publishing;1993. p. 40.
5. Nap A, Balt JC, Mathy MJ, Van Zwieten PA. AT (1)-receptor blockade and sympathetic neurotransmission in cardiovascular disease. Auton Autacoid Pharmacol. 2003; 23:285–96.
6. Feldberg W, Lewis GP. The action of peptides on the adrenal medulla: release of adrenaline by bradykinin and angiotensin. J Physiol. 1964; 171:98–108.

7. Chulak C, Couture R, Foucart S. Modulatory effect of bradykinin on the release of noradrenaline from rat isolated atria. Br J Pharmacol. 1995; 115:330–4.

8. Rump LC, Berlit T, Schwertfeger E, Beyersdorf F, Schollmeyer P, Bohmann C. Angiotensin converting enzyme inhibition unmasks the sympathofacilitatory effect of bradykinin in human right atrium. J Hypertens. 1997; 15:1263–70.

9. Starke K, Peskar BA, Schumacher KA, Taube HD. Bradykinin and postganglionic sympathetic transmission. Naunyn Schmiedebergs Arch Pharmacol. 1977; 299:23–32.

10. Franchi F, Lazzeri C, Foschi M, Tosti-Guerra C, Barletta G. Cardiac autonomic tone during trandolapril-irbesartan low-dose combined therapy in hypertension: a pilot project. J Hum Hypertens. 2002; 16:597–604.

11. Karas M, Lacourciere Y, LeBlanc AR, Nadeau R, Dube B, Florescu M, et al. Effect of the renin-angiotensin system or calcium channel blockade on the circadian variation of heart rate variability, blood pressure and circulating catecholamines in hypertensive patients. J Hypertens. 2005; 23:1251–60.

12. Sakata K, Yoshida H, Obayashi K, Ishikawa J, Tamekiyo H, Nawada R, et al. Effects of losartan and its combination with quinapril on the cardiac sympathetic nervous system and neurohormonal status in essential hypertension. J Hypertens. 2002; 20:103–10.

13. Balt JC, Mathy MJ, Pfaffendorf M. van Zwieten PA. Inhibition of angiotensin II-induced facilitation of sympathetic neurotransmission in the pithed rat: a comparison between losartan, irbesartan, telmisartan, and captopril. J Hypertens. 2001; 19:465–73.
14. Randa HD. Renin and angiotensin. Brunton LL, Bruce AC, Bjorn CK, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill Health Publishing Division;2011:p. 721–44.
15. Ball SG, White WB. Debate: angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers–a gap in evidence-based medicine. Am J Cardiol. 2003; 91(10A):15G–21G.

16. Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981; 313:463–80.

17. Anton AH, Sayre DF. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962; 138:360–75.
18. Tallarida RJ, Murray RB. Manual of pharmacologic calculation with computer programs. 2nd ed. New York: Springer-Verlag. 1987; 110:131–6.
19. Hammer R, Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982; 31:2991–8.

20. Hano T, Mizukoshi M, Baba A, Nakamura N, Nishio I. Angiotensin II subtype 1 receptor modulates epinephrine release from isolated rat adrenal gland. Blood Press Suppl. 1994; 5:105–8.
21. Garcia AG, Sala F, Reig JA, Viniegra S, Frias J, Fonteriz R, et al. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984; 309:69–71.

22. Lim DY, Kim CD, Ahn KW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 1992; 15:115–25.

23. Goeger DE, Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989; 38:3995–4003.
24. Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989; 264:17816–23.

25. Wada Y, Satoh K, Taira N. Cardiovascular profile of Bay K 8644, a presumed calcium channel activator, in the dog. Naunyn Schmiedebergs Arch Pharmacol. 1985; 328:382–7.

26. Martineau D, Yamaguchi N, Briand R. Inhibition by BMS 186295, a selective nonpeptide AT1 antagonist, of adrenal catecholamine release induced by angiotensin II in the dog in vivo. Can J Physiol Pharmacol. 1995; 73:459–64.
27. Martineau D, Briand R, Yamaguchi N. Functional evidence for L-type Ca2+ channels controlling ANG II-induced adrenal catecholamine release in vivo. Am J Physiol. 1996; 271(6 Pt 2):R1713–9.

28. Martineau D, Lamouche S, Briand R, Yamaguchi N. Functional involvement of angiotensin AT2 receptor in adrenal catecholamine secretion in vivo. Can J Physiol Pharmacol. 1999; 77:367–74.

29. Yamaguchi N, Martineau D, Lamouche S, Briand R. Functional role of local angiotensin-converting enzyme (ACE) in adrenal catecholamine secretion in vivo. Can J Physiol Pharmacol. 1999; 77:878–85.

30. Cavadas C, Grand D, Mosimann F, Cotrim MD, Fontes Ribeiro CA, Brunner HR, et al. Angiotensin II mediates catecholamine and neuropeptide Y secretion in human adrenal chromaffin cells through the AT1 receptor. Regul Pept. 2003; 111:61–5.
31. Critchley L, Ding B, Fok B, Wang D, Tomlinson B, James A, et al. The effects of candesartan and ramipril on adrenal cat-echolamine release in anaesthetized dogs. Eur J Pharmacol. 2004; 489:67–75.

32. Koji T, Onishi K, Dohi K, Okamoto R, Tanabe M, Kitamura T, et al. Addition of angiotensin II receptor antagonist to an ACE inhibitor in heart failure improves cardiovascular function by a bradykinin-mediated mechanism. J Cardiovasc Pharmacol. 2003; 41:632–9.

33. Akat PB, Bapat TR, Murthy MB, Karande VB, Burute SR. Comparison of the efficacy and tolerability of telmisartan and enalapril in patients of mild to moderate essential hypertension. Indian J Pharmacol. 2010; 42:153–6.

34. Zou Z, Xi GL, Yuan HB, Zhu QF, Shi XY. Telmisartan versus angiotension-converting enzyme inhibitors in the treatment of hypertension: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2009; 23:339–49.

35. Lewandowski J, Abramczyk P, Dobosiewicz A, Bidiuk J, Sinski M, Gaciong Z. The effect of enalapril and telmisartan on clinical and biochemical indices of sympathetic activity in hypertensive patients. Clin Exp Hypertens. 2008; 30:423–32.

36. Smith DH. Treatment of hypertension with an angiotensin II-receptor antagonist compared with an angiotensin-converting enzyme inhibitor: a review of clinical studies of telmisartan and enalapril. Clin Ther. 2002; 24:1484–501.

37. Schramm M, Thomas G, Towart R, Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983; 303:535–7.

38. Fisher SK, Holz RW, Agranoff BW. Muscarinic receptors in chromaffin cell cultures mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 1981; 37:491–7.

39. Yanagihara N, Isosaki M, Ohuchi T, Oka M. Muscarinic receptor-mediated increase in cyclic GMP level in isolated bovine adrenal medullary cells. FEBS Lett. 1979; 105:296–8.

40. Wakade AR, Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience. 1983; 10:973–8.

41. Lim DY, Hwang DH. Studies on secretion of catecholamines evoked by DMPP and McN-A-343 in the rat adrenal gland. Korean J Pharmacol. 1991; 27:53–67.
42. Kilpatrick DL, Slepetis RJ, Corcoran JJ, Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 1982; 38:427–35.

43. Kilpatrick DL, Slepetis RJ, Corcoran JJ, Kirshner N. Calcium uptake and catecholamine secretion by cultured bovine adrenal medulla cells. J Neurochem. 1982; 38:427–35.

44. Knight DE, Kesteven NT. Evoked transient intracellular free Ca2+ changes and secretion in isolated bovine adrenal medullary cells. Proc R Soc Lond B Biol Sci. 1983; 218:177–99.
45. Wada A, Takara H, Izumi F, Kobayashi H, Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience. 1985; 15:283–92.

46. Kidokoro Y, Ritchie AK. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980; 307:199–216.

47. Burgoyne RD. Mechanisms of secretion from adrenal chromaffin cells. Biochim Biophys Acta. 1984; 779:201–16.

48. Oka M, Isosaki M, Yanagihara N. Isolated bovine adrenal medullary cells: studies on regulation of catecholamine synthesis and release. Usdin E, Kopin IJ, editors. Catecholamines: basic and clinical frontiers. Oxford: Pergamon Press;1979:p. 70–2.

49. Suzuki M, Muraki K, Imaizumi Y, Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca(2+)-pump, reduces Ca(2+)-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992; 107:134–40.

50. Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989; 94:363–83.

51. Uyama Y, Imaizumi Y, Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992; 106:208–14.

52. Cheek TR, O’Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells: distinct nicotinic and muscarinic patterns. FEBS Lett. 1989; 247:429–34.
53. Challis RA, Jones JA, Owen PJ, Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5- tetraki-sphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 1991; 56:1083–6.
54. Azizi M, Guyene TT, Chatellier G, Wargon M, Menard J. Additive effects of losartan and enalapril on blood pressure and plasma active renin. Hypertension. 1997; 29:634–40.

55. Raasch W, Johren O, Schwartz S, Gieselberg A, Dominiak P. Combined blockade of AT1-receptors and ACE synergistically potentiates antihypertensive effects in SHR. J Hypertens. 2004; 22:611–8.
56. Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment on left ventricular mass in essential hypertension. Am J Med. 2003; 115:41–6.

57. Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000; 102:1388–93.

58. Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002; 105:2512–7.

59. Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004; 110:1456–62.

60. Suzuki H, Kanno Y, Kaneko K, Kaneko M, Kotaki S, Mimura T, et al. Comparison of the effects of angiotensin receptor antagonist, angiotensin converting enzyme inhibitor, and their combination on regression of left ventricular hypertrophy of diabetes type 2 patients on recent onset hemodialysis therapy. Ther Apher Dial. 2004; 8:320–7.

Go to : 
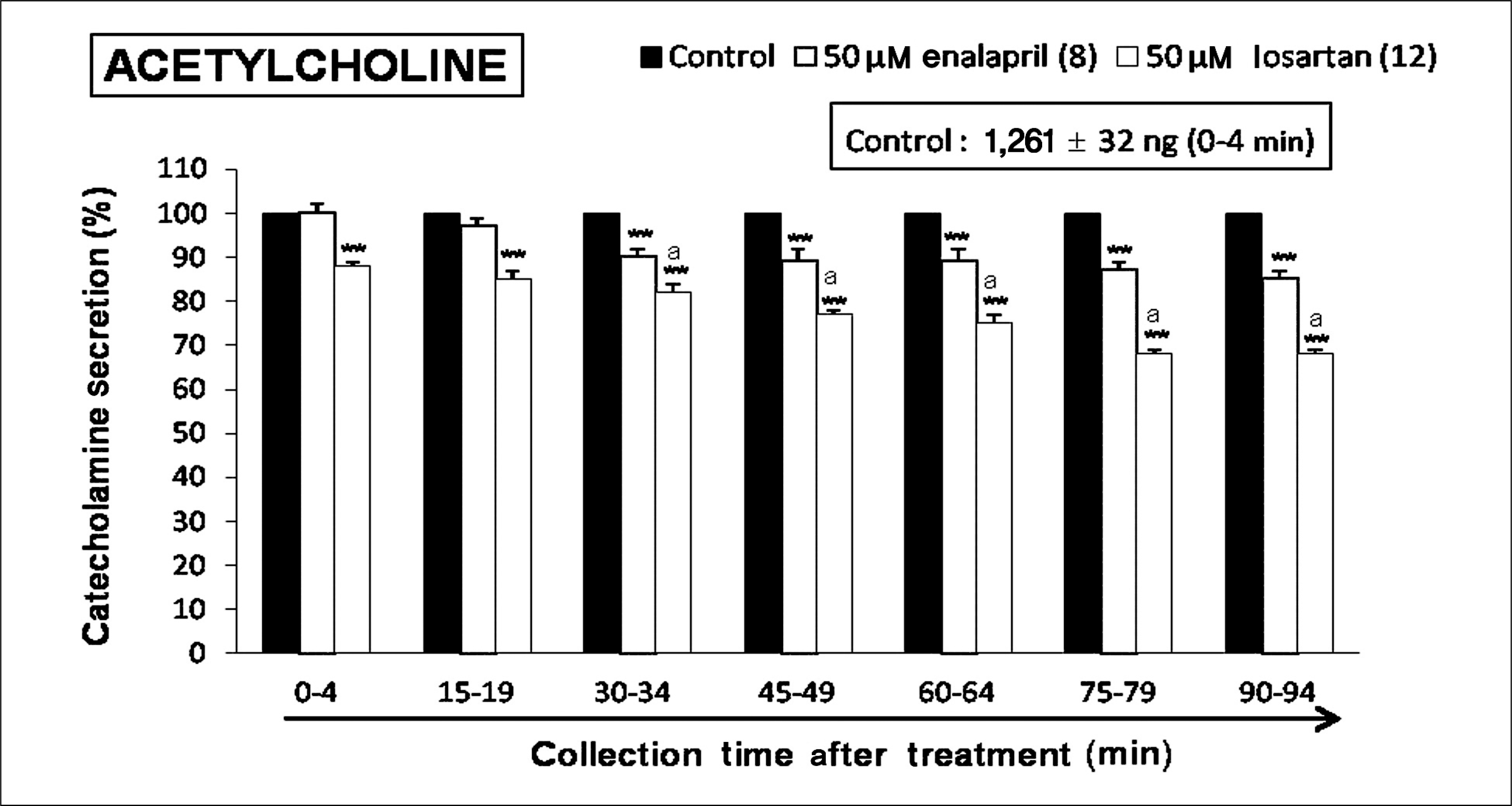 | Fig. 1.Time-course effects of losartan or enalapril on the secretory responses of CA evoked by acetylcholine from the isolated perfused rat adrenal glands. The CA secretion by a single injection of (ACh, 5.32 × 10-3 M) in a volume of 0.05 mL was evoked at 15 minutes intervals during loading with losartan (50 μM) or enalapril (50 μM) for 90 minutes as indicated by the arrow marks. Columns and vertical lines represent mean ± standard error. Numbers in the parenthesis indicate number of rat adrenal glands. Ordinate: the amounts of CA secreted from the adrenal gland (% of control). Abscissa: collection time of perfusate (minutes). Statistical difference was obtained by comparing the corresponding control with losartan- or enalapril-treated group (**), and by comparing the losartan-treated group with enalapril-treated group (a). ACh-induced perfusates were collected for 4 minutes. **p < 0.01, ap < 0.05. CA, catecholamines; ACh, acetylcholine. |
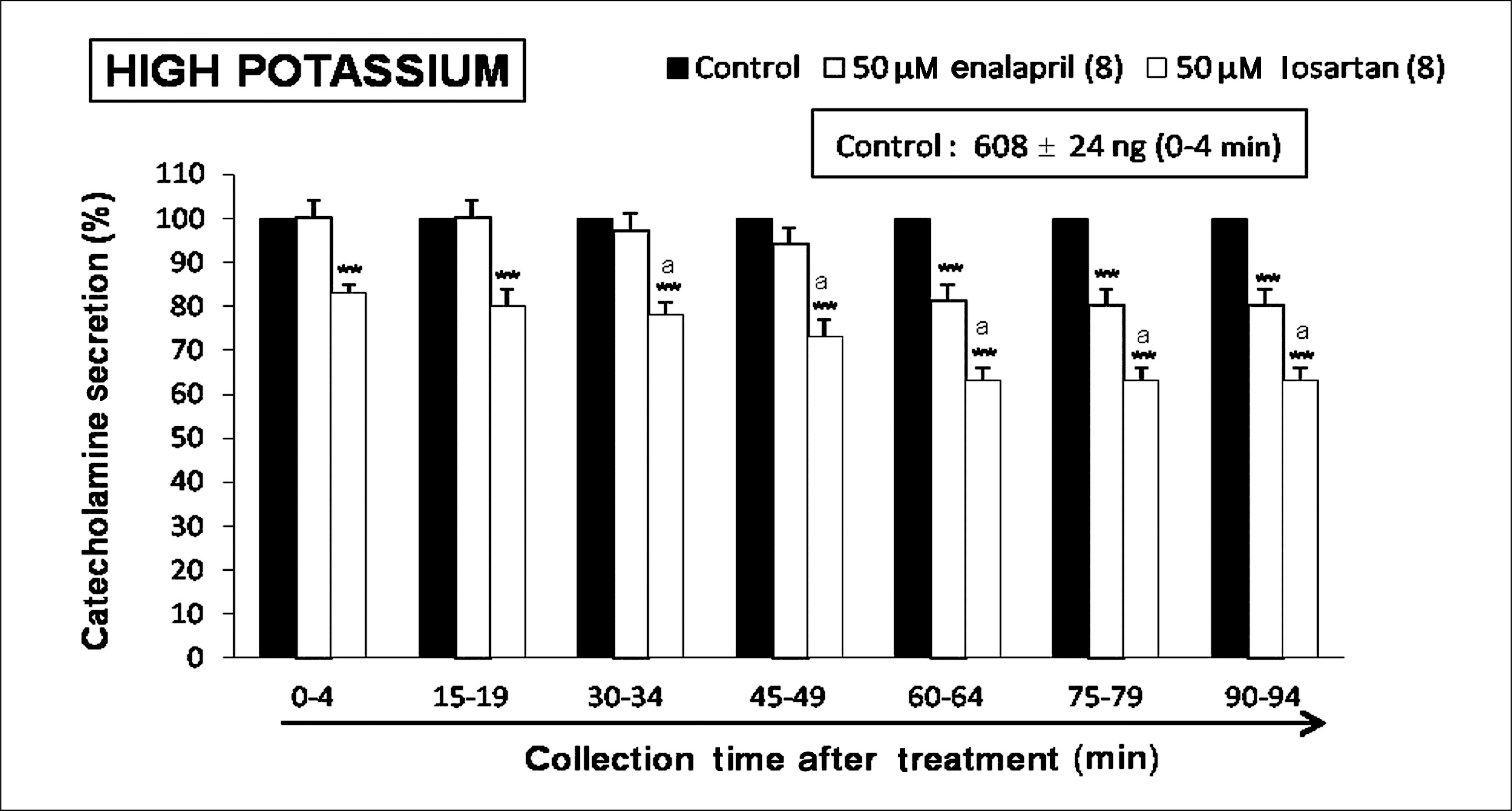 | Fig. 2.Time-course effects of losartan or enalapril on high potassium-evoked catecholamines (CA) secretion from the isolated perfused rat adrenal glands. The CA secretion by a single injection of high potassium (56 mM) in a volume of 0.05 mL was evoked at 15 minutes intervals during loading with losartan (50 μM) or enalapril (50 μM) for 90 minutes as indicated by the arrow mark. Other legends are the same as in Fig. 1. High potassium-induced perfusates were collected for 4 minutes. **p < 0.01, ap < 0.05. |
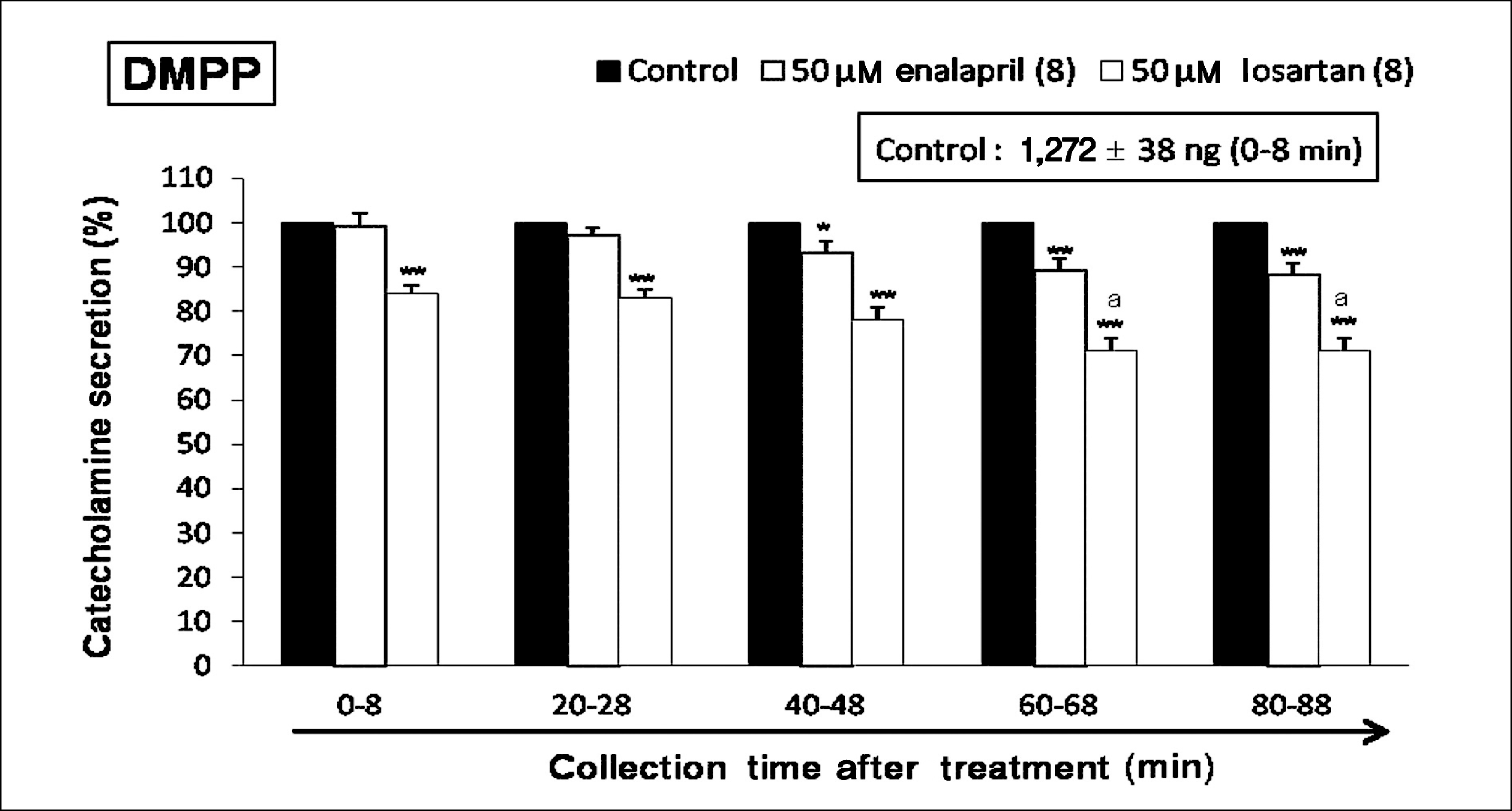 | Fig. 3.Time-course effects of losartan or enalapril on 1.1-dimethyl-4 -phenyl piperazinium iodide (DMPP)-evoked catecholamines (CA) secretion from the isolated perfused rat adrenal glands. The CA secretion by perfusion of DMPP (10-4 M) for 2 minutes was induced at 20 minutes intervals during loading with losartan (50 μM) or enalapril (50 μM) for 90 minutes as indicated by the arrow mark. DMPP-induced perfusates were collected for 8 minutes. Other legends are the same as in Fig. 1.*p < 0.05, **p < 0.01, ap < 0.05. |
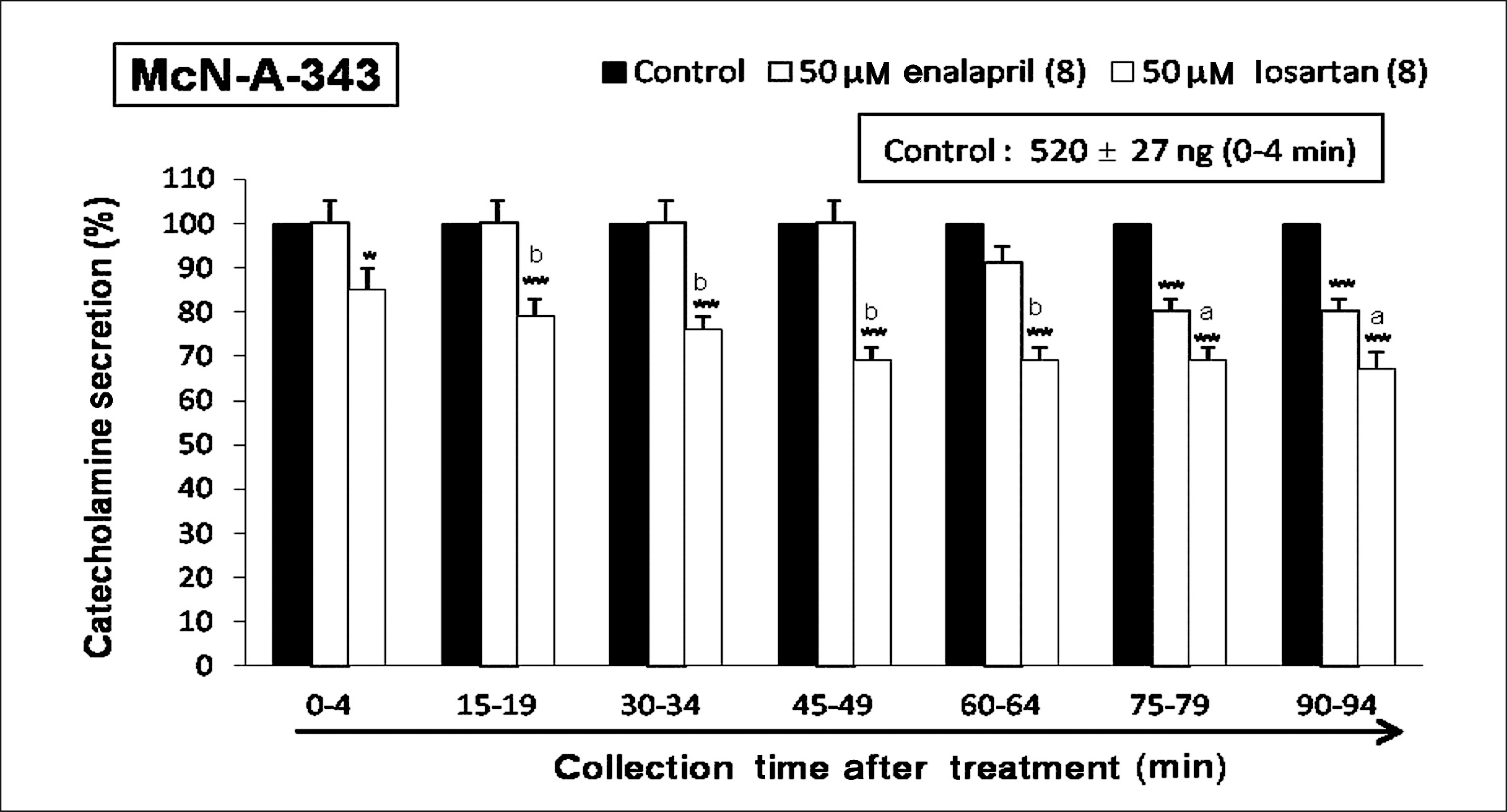 | Fig. 4.Time-course effects of losartan or enalapril on McN-A-343-evoked catecholamines (CA) secretion from the isolated perfused rat adrenal glands. The CA secretion by perfusion of 3-(m-chlloro-phenyl- carbamoyl-oxy-2-butynyl-trimethyl ammonium chloride (McN-A-343, 10-4 M) for 4 minutes was induced at 15 minutes intervals during loading with losartan (50 μM) or enalapril (50 μM) for 90 minutes as indicated by the arrow mark. McN-A-343-induced perfusates were collected for 4 minutes. Other legends are the same as in Fig. 1. *p < 0.05, **p < 0.01. ap< 0.05, bp < 0.01. |
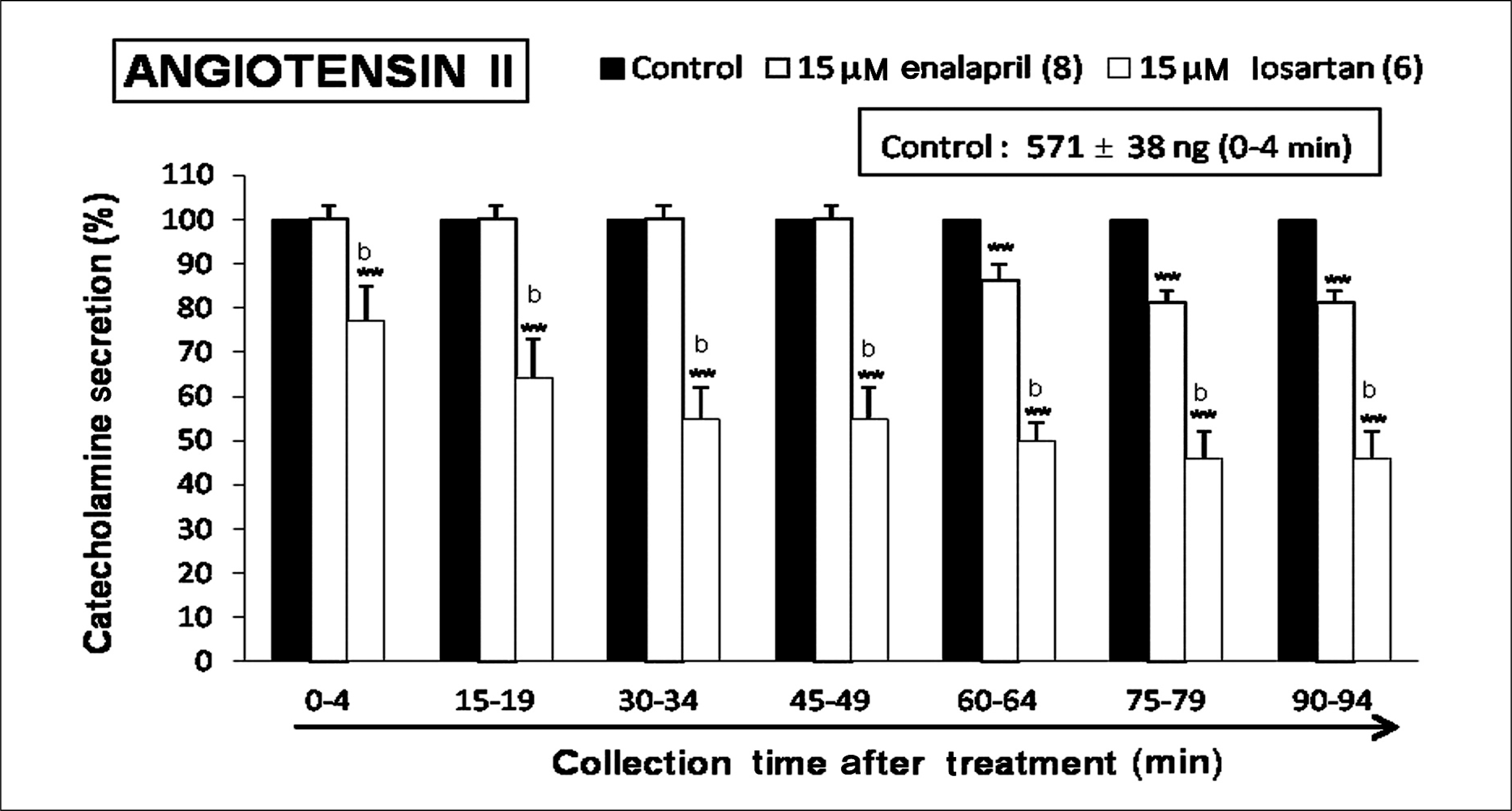 | Fig. 5.Time-course effects of losartan or enalapril on angiotensin II-evoked catecholamines (CA) secretion from the perfused rat adrenal glands. The CA secretion by perfusion of angiotensin II (10-7 M) for 4 minutes was induced at 15 minutes intervals during loading with losartan (15 μM) or enalapril (15 μM) for 90 minutes as indicated by the arrow mark. Angiotensin II-induced perfusates were collected for 4 minutes. Other legends are the same as in Fig. 1. **p < 0.01. bp < 0.01. |
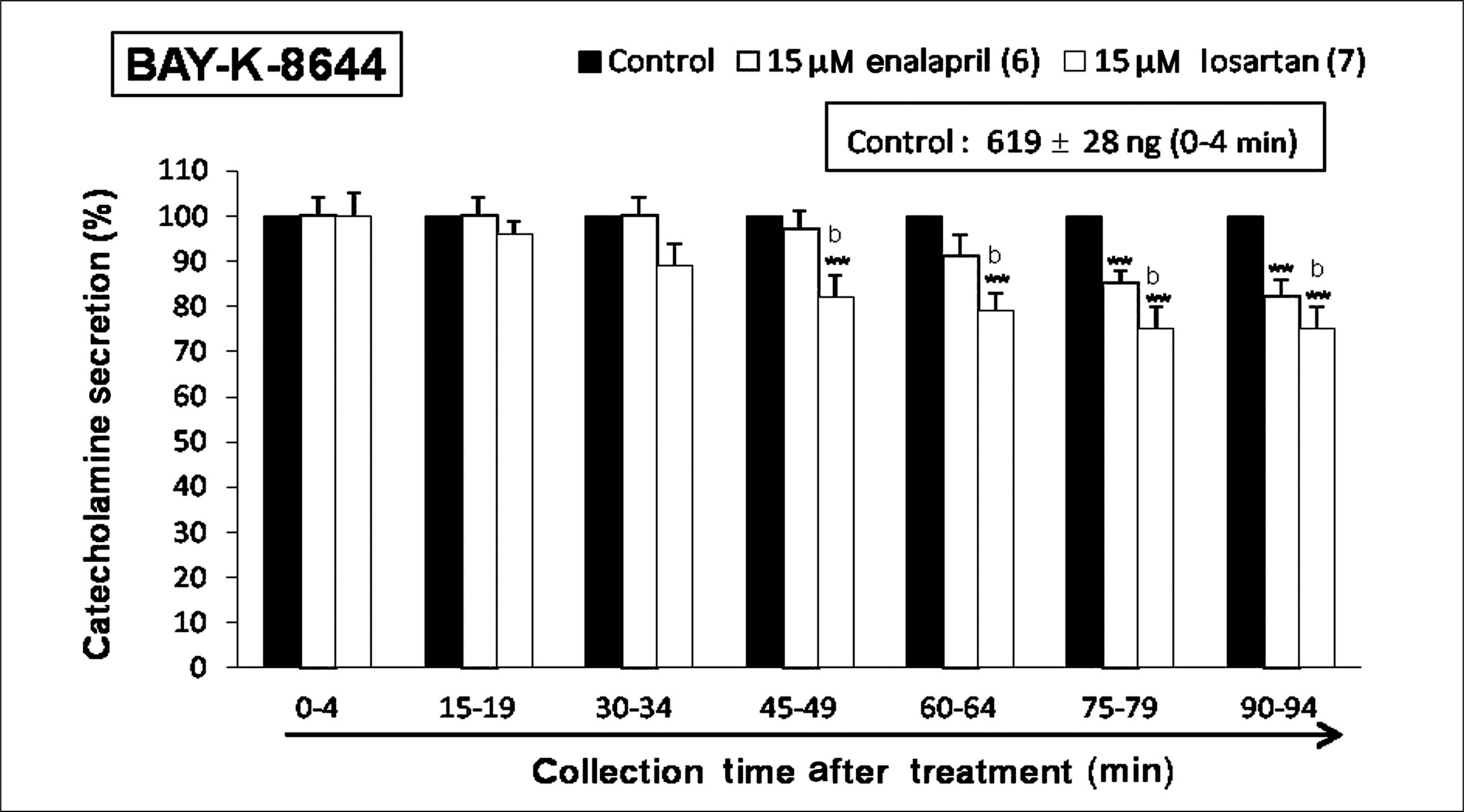 | Fig. 6.Time-course effects of losartan or enalapril on 6-dimethyl-3-nitro-4-(2-trifluoromethyl-phenyl)-pyridine-5-carboxylate (BAY-K-8644)-evoked catecholamines (CA) secretion from the perfused rat adrenal glands. The CA secretion by perfusion of BAY-K-8644 (10-5 M) for 4 minutes was induced at 15 minutes intervals during loading with losartan (15 μM) or enalapril (15 μM) for 90 minutes as indicated by the arrow mark. Bay-K-8644-induced perfusates were collected for 4 minutes. Other legends are the same as in Fig. 1. **p < 0.01, bp < 0.01. |
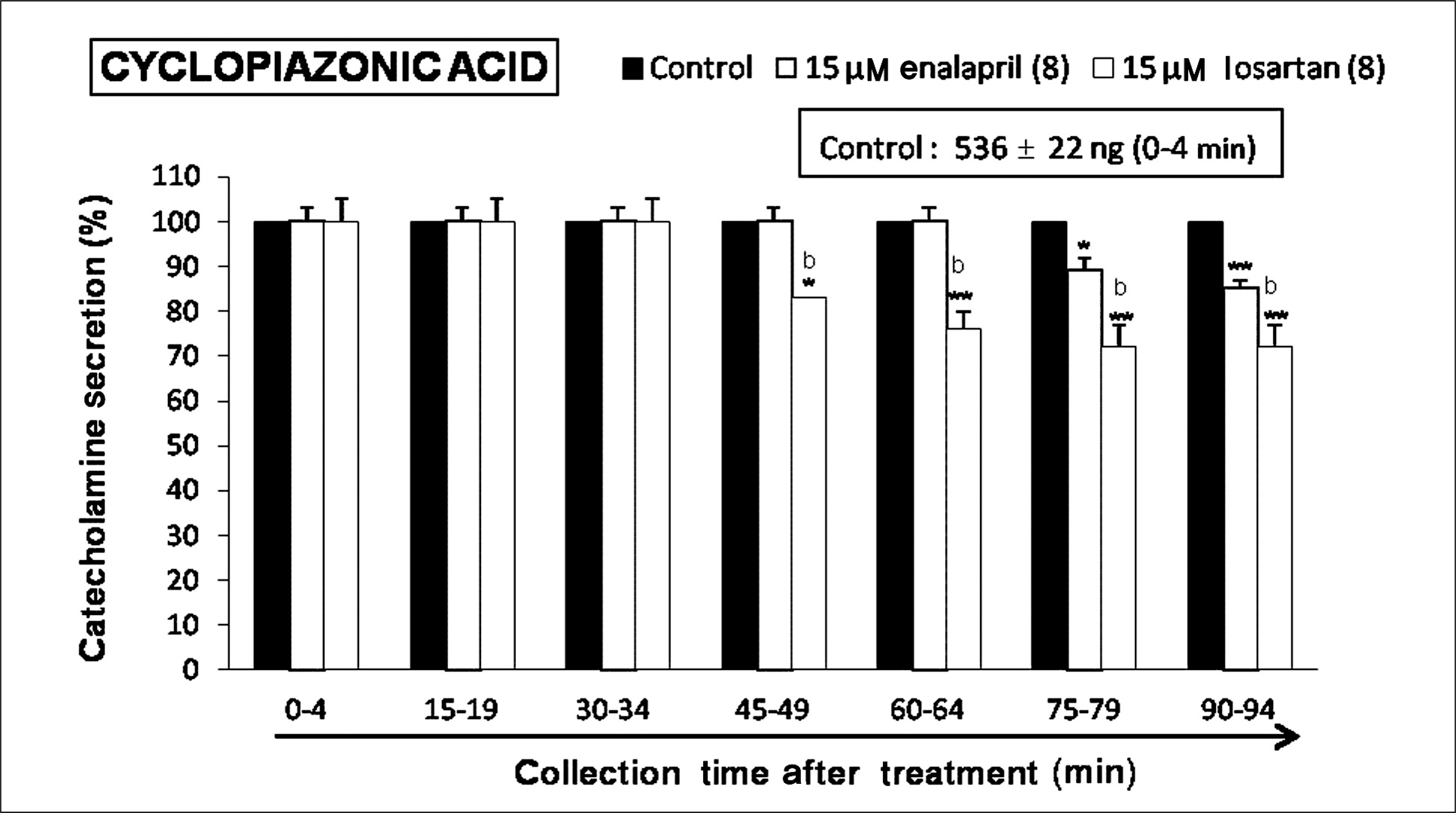 | Fig. 7.Time-course effects of losartan or enalapril on cyclopiazonic acid-evoked catecholamines (CA) secretion from the perfused rat adrenal glands. The CA secretion by perfusion of cyclopiazonic acid (10-5 M) for 4 minutes was induced at 15 minutes intervals during loading with losartan (15 μM) or enalapril (15 μM) for 90 minutes as indicated by the arrow mark. Cyclopiazonic acid-induced perfusates were collected for 4 minutes. Other legends are the same as in Fig. 1. *p < 0.05, **p < 0.01, bp < 0.01. |
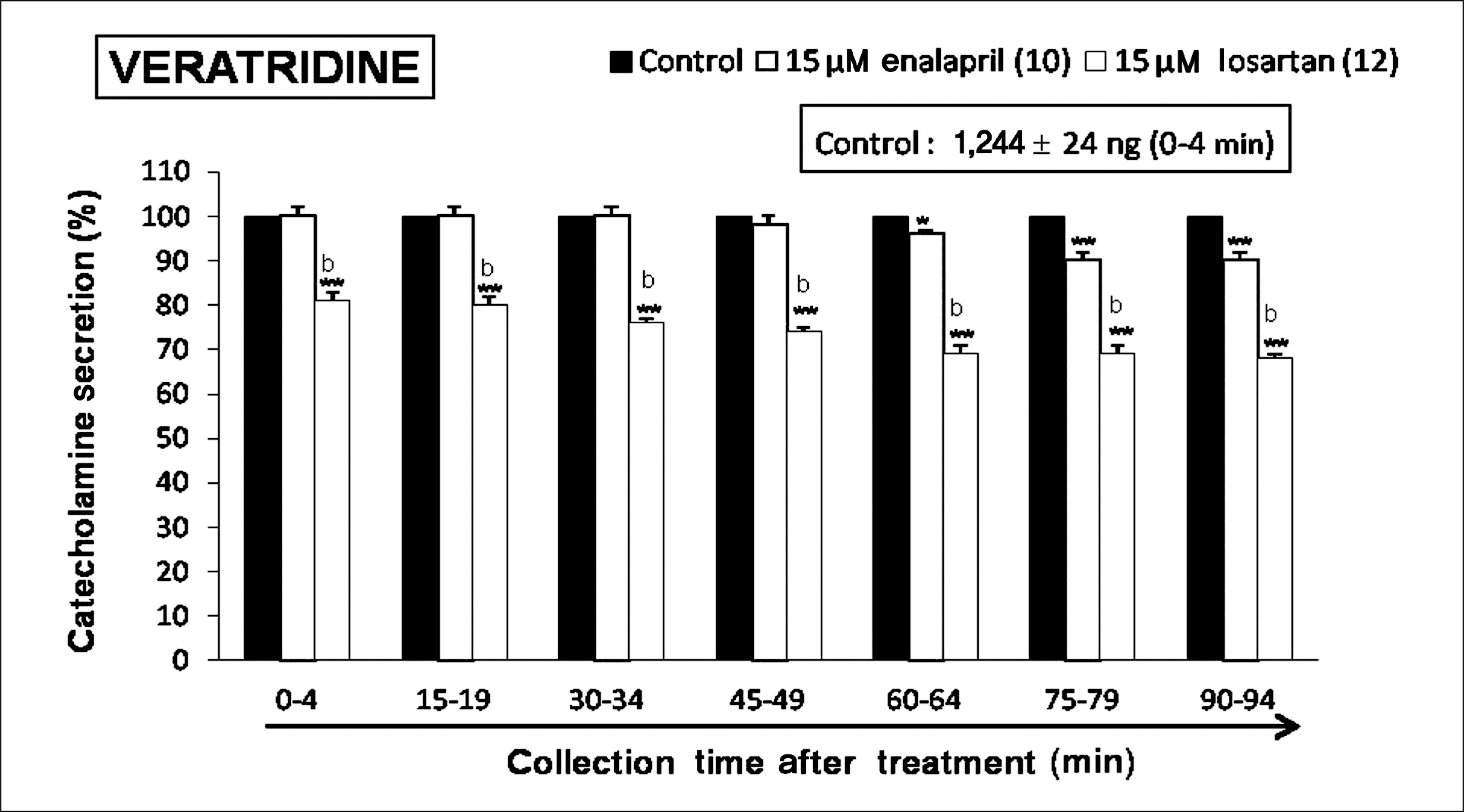 | Fig. 8.Time-course effects of losartan or enalapril on veratridine-evoked catecholamines (CA) secretion from the perfused rat adrenal glands. The CA secretion by perfusion of veratridine (10-5 M) for 4 minutes was induced at 15 minutes intervals during loading with losartan (15 μM) or enalapril (15 μM) for 90 minutes as indicated by the arrow mark. Veratridine-induced perfusates were collected for 4 minutes. Other legends are the same as in Fig. 1. *p < 0.05, **p < 0.01, bp < 0.01. |
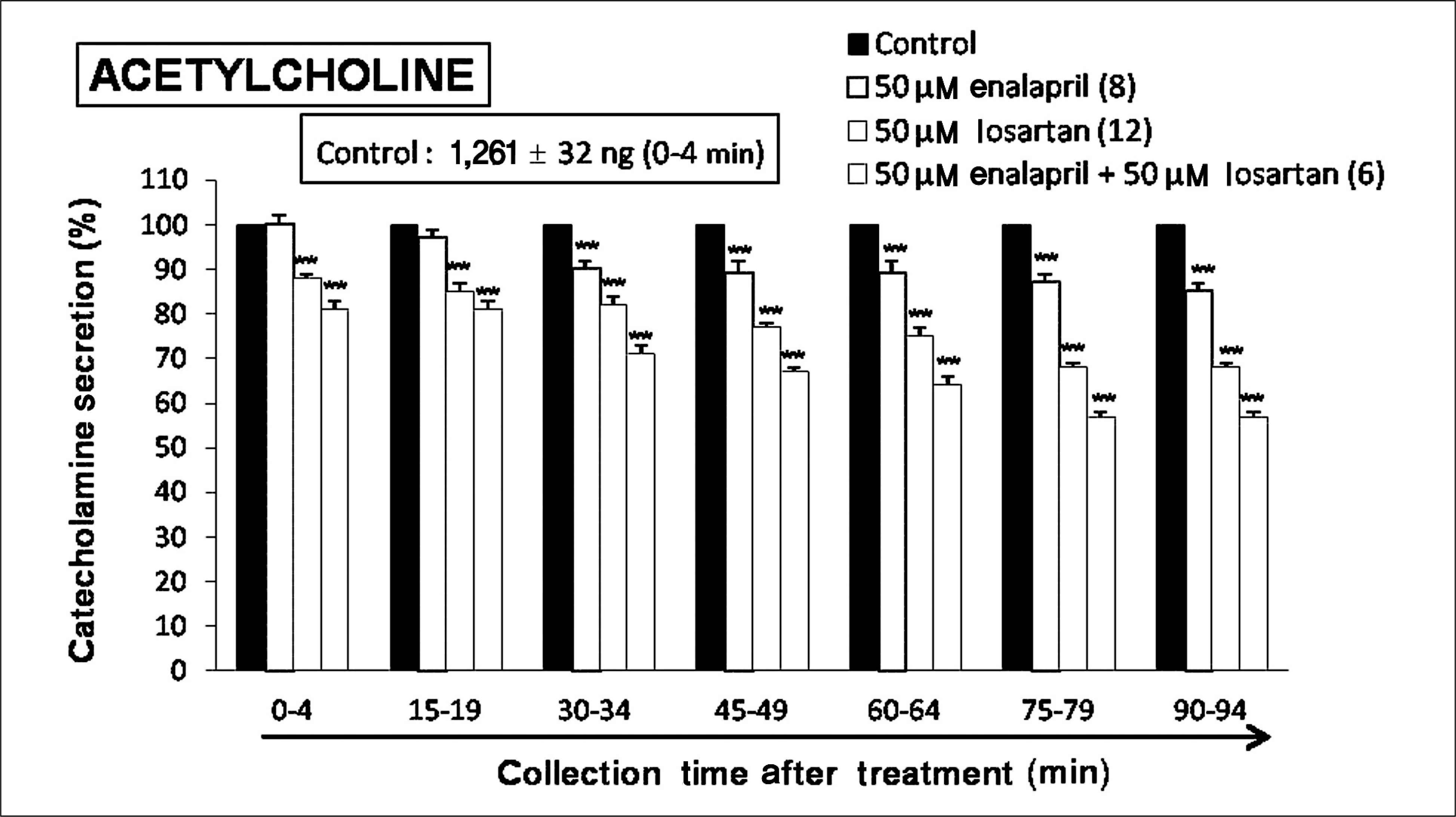 | Fig. 9.Comparative time-course effects of losartan, enalapril and losartan + enalapril on acetylcholine-evoked catecholamines (CA) secretion from the perfused rat adrenal glands. The CA secretion by a single injection of acetylcholine chloride (5.32 × 10-3 M) in a volume of 0.05 mL was evoked at 15 minutes intervals during loading with losartan (50 μM) or enalapril (50 μM), losartan (50 μM) + enalapril (50 μM) for 90 minutes as indicated by the arrow mark. Acetlcholine-induced perfusates were collected for 4 minutes. Other legends are the same as in Fig. 1. **p < 0.01 enalapril-treated group vs. losartan-treated group or enalapril + losartan-treated group. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download